|
Home
Prof. Newcomb
Research Group
Research Projects
Facilities
Publications
Research advances are at the link below
Advances
|
|
Research projects are in the broad areas of physical
organic chemistry and biochemistry.
Projects range from laser flash
photolysis kinetic studies to mechanistic studies with enzymes.
Follow
the links below for more information.
|
|
|
|
|
|
|
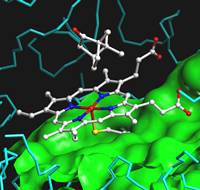
The active site of P450(cam) with its substrate, camphor. The fifth ligand to heme iron is thiolate from cysteine in the protein (yellow).
|
|
Cytochrome P450 Enzymes The ubiquitous cytochrome P450 enzymes catalyze
a wide range of biological oxidations including in the remarkably
difficult hydroxylation of hydrocarbons. Our research with
cytochromes P450 focuses on the mechanism the hydroxylation reactions
and the nature of the active oxidants. Recent work has confirmed
that two active electrophilic oxidant forms are produced in P450s, an
iron-oxo species that has long been thought to be an oxidant, and a
precursor to this species, either a hydroperoxy-iron intermediate or
iron-complexed hydrogen peroxide. The studies employed a highly
precise GC-MS protocol using CI, and different kinetic isotope effects
were found for two products formed by oxidation of the same position on
a mechanistic probe. Current work is focused on isolating the two
oxidants via their kinetics. Related work includes mechanistic
studies of NO-synthase enzymes, the heme-containing enzymes that produce
nitric oxide by oxidation of arginine. TOP |
| |
|
|
|
|
|
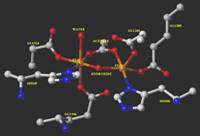
The active site of an sMMO hydroxylase enzyme. The iron atoms are
orange. |
|
Methane Monooxygenase Enzymes The methanotrophic bacteria use
methane as a source of carbon. The first functionalization
reaction, conversion of methane to methanol, is one of the most
difficult reactions effected in nature. These oxidations are
executed by methane monooxygenase (MMO) enzymes. Soluble MMO
enzymes have been crystallized, and their structures determined by X-ray
crystallography. They contain a diiron active site as shown in the
picture. The MMO reaction cycle is related to the P450 reaction
cycle in that oxygen is the reduced to give the equivalent of hydrogen
peroxide. Also like P450, the mechanisms of the MMO hydroxylation
reactions are disputed. We are studying the kinetics of MMO
oxidations directly via stopped-flow spectroscopy and indirectly with
mechanistic probes. TOP |
| |
|
|
|
| |
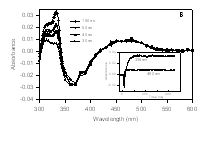
|
|
Radical Kinetics A number of radical kinetic studies are
ongoing in the laboratory. Most employ laser flash photolysis
methods wherein the radical is produced by a laser flash, and radicals
with UV chromophores are monitored. The plot at the left shows a
time-resolved spectrum obtained from a reaction of an enol ether radical
cation. The product contains a chromophore at 335 nm that is
growing in with time. The inset shows the formation of an
"instantaneous" signal at 490 nm from a by-product radical produced by
the laser flash and the growing signal at 335 nm from the product, a
distonic radical cation with a diphenylalkyl radical moiety.
TOP |
| |
|
|
|
| |
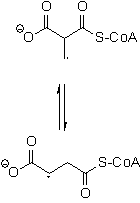
|
|
Enzyme-Catalyzed Radical Rearrangements Coenzyme B12-dependent
enzymes catalyze radical rearrangements in nature involving a 1,2-shift
of a group. A typical example is the rearrangement of
methylmalonyl-CoA to succinyl-CoA catalyzed by methylmalonyl-CoA mutase.
We are studying the mechanisms of these reactions by laser flash
photolysis kinetic studies of model reactions. A premise of our
program is that the mechanisms of a wide range of rearrangements could
be similar, involving polar effects in the radical reactions. Our
studies are unique in that we can produce the radicals in polar media
including water. Evaluation of the kinetics of the radical
rearrangements as a function of pH will allow one to determine whether
acid or base catalysis is important. The program involves
synthetic work to produce the model radical precursors, laser flash
photolysis studies, computational studies of the reactions, and enzyme
modeling. TOP |
| |
|
|
|
|