NMR Analysis of Chloroform
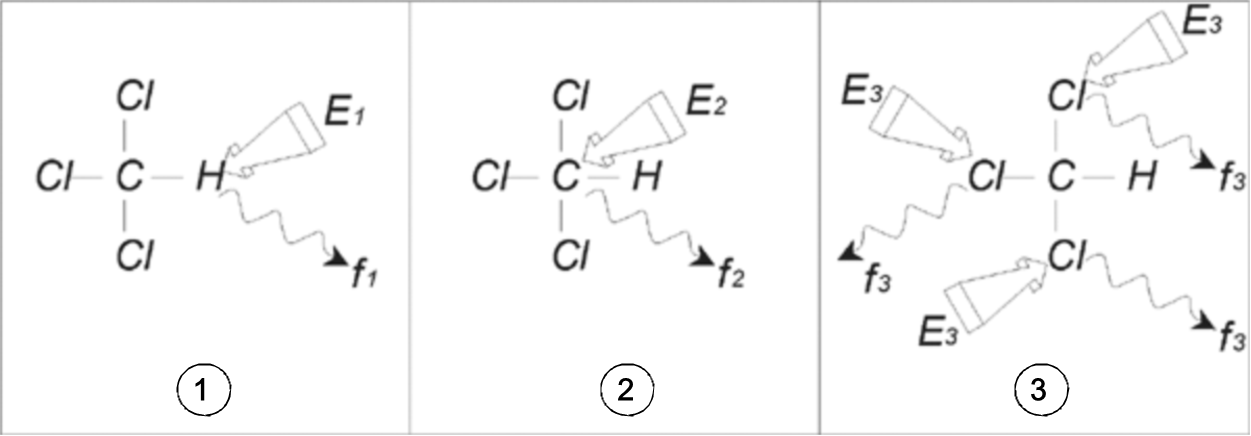
Three separate experiments, as outlined in the figure below, can be performed corresponding to the three possible observe nuclei 1H, 13C and 35Cl.
NMR Analysis of CHCI3
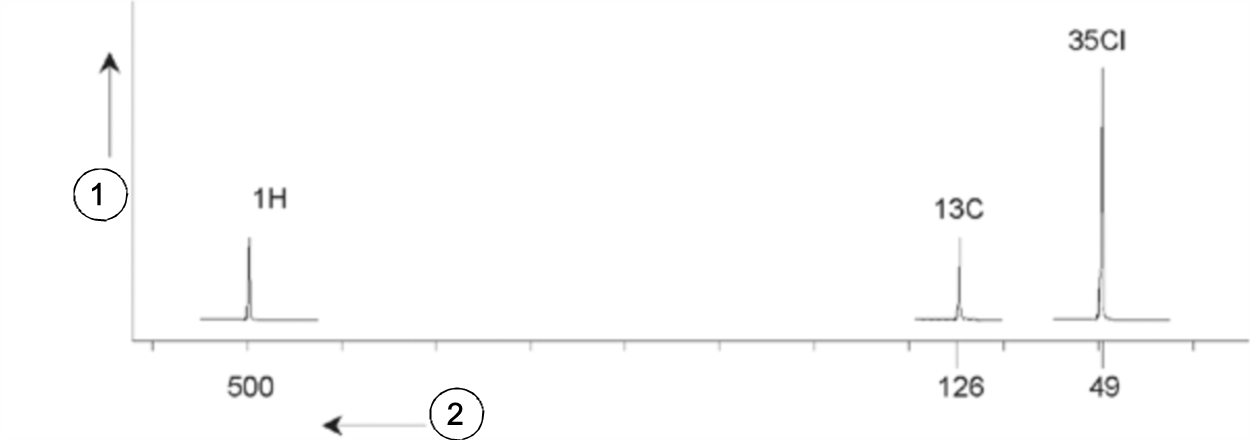
Three excitation pulses (E1, E2, E3) are directed at the sample at appropriate carrier frequencies. E1 corresponds to the 1H resonance frequency, E2 to the 13C frequency, and E3 to the 35Cl frequency. Assuming the three isotopes were successfully excited, the sample will emit signals at three frequencies f1, f2 and f3 which are recorded on three separate spectra. If the emitted signals are displayed in a single plot, the user can expect a spectrum similar to that in the figure below (note that the signal frequencies illustrated are for a 11.7 T magnet and that all signals have been plotted as singlets, i.e. single peaks).
This artificial spectrum shows three peaks corresponding to the three isotopes. Taking the relative numbers of the three isotopes into account, one might expect that the intensity of the chlorine, hydrogen and carbon peaks would be in the ratio of 3:1:1. However, the natural abundance of the three isotopes must also be accounted for, resulting in a ratio of 227:100:1. The user will find that the experimentally determined peak intensity ratios will not agree with these values. The reason is that every isotope has an inherent sensitivity to the NMR technique. The 1H is 63 times more sensitive to NMR than 13C. Thus, even if a sample would contain exactly the same number of 1H nuclei as 13C, the intensity of the 1H signals will be 63 times greater than the 13C signals.
With a plot such as that in the figure above, any detailed information will be lost and precise determination of a particular frequency would be impossible. The spectrum would be said to display very poor resolution (the horizontal resolution of a spectrum is a measure of how well the spectrum differentiates between two signals that are close in frequency).
A further complication is the huge range in vertical scaling. The variation in inherent sensitivity to NMR, coupled with the variations in natural abundance, will often make the plotting of signals from different isotopes onto a single plot unfeasible. In fact, the vertical resolution of the spectrum will be very poor (the vertical resolution, i.e. the signal to noise ratio of a spectrum is a measure of sensitivity).
If our analysis of chloroform is proving rather complicated, it is because we are attempting to compare the signals from three different observe nuclei on a single spectrum (we are ignoring here any hardware/electronic restrictions). Therefore, in practice, NMR experiments are performed with a single observe nucleus. Although more than one isotope may be excited simultaneously, by using more than one carrier frequency (e.g. decoupling experiments), we only ever observe the signals from a single isotope. This greatly simplifies spectrum analysis.
It was mentioned earlier that variations in the basic resonance frequency due to the local atomic environment tend to be relatively small. Thus, large spectral ranges will not be encountered. Furthermore natural abundance and inherent sensitivity will always be the same for a particular isotope. Hence, the relative intensity of say two signals emitted from 1H isotopes on a single spectrum will depend only on the number of atoms contributing to the signal. This greatly simplifies analysis of spectra for quantitative information. Before proceeding further with a more detailed description of NMR the reader should become familiar with the concept of measuring signals in ppm (parts per million) with respect to a reference signal.
| Glossary entry: | ppm |
Parts Per Million