Proton Spectrum of Ethylbenzene with Spin/Spin Coupling
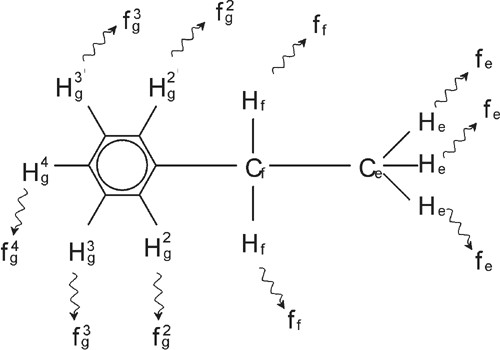
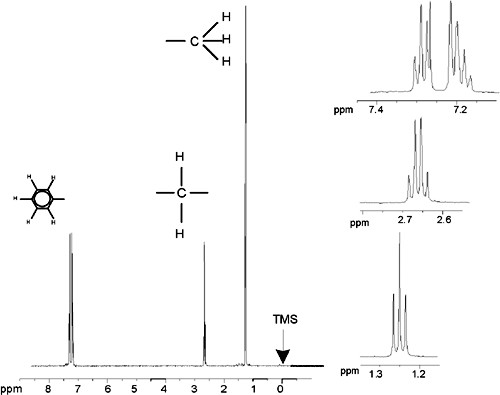
The description of proton NMR spectra thus far has been greatly simplified by the fact that all signals, with the exception of those from the benzene ring in benzylacetate, have been singlets. The structure of the organic compound ethylbenzene and the corresponding proton spectrum are illustrated in the Ethylbenzene figure and the Ethylbenzene Spectrum figure respectively. As before, the protons have been labeled as three distinct groups corresponding to three basic atomic environments.
The most obvious difference between the signals in this spectrum and those of benzylacetate is the splitting into multiplets. The signal emitted by the CH3 protons is a triplet and the signal from the CH2 protons is a quartet. Note also that the signal positions do not coincide. The CH3 protons in benzylacetate emit a signal at 1.85 ppm, while the corresponding CH3 protons in ethylbenzene emit the triplet signal at 1.25 ppm. This is hardly surprising, because the two CH3 groups are in different chemical environments.
The cause of the multiplet splitting is due to an effect known as spin-spin coupling. A full account of this effect is beyond the scope of this manual and the reader should refer to a standard NMR text for details. For our purpose a brief outline of the spin-spin coupling should suffice.
Ethylbenzene
Ethylbenzene Spectrum
The splitting of NMR signals in the figure Ethylbenzene results from a magnetic interaction between neighboring protons. The two Hf protons are magnetically equivalent and do not interact with each other. Similarly, the three He protons are magnetically equivalent and do not affect each other. However, the two Hf protons and the three He protons are in different local environments and are “coupled” to each other via their bonding electrons. The net result of this coupling is that the two groups of protons interact with each other and cause the splitting of the NMR signals.
The two Hf protons can combine to exist in three possible magnetic states (this is a result of spin orientation and hence the term spin-spin coupling). As a result of coupling, the NMR signals emitted by the He protons resonate at three possible frequencies and a triplet is observed.
Similarly, the effect of the He protons is to split the Hf signals. The three He protons can combine to exist in four possible magnetic states. Consequently the Hf protons resonate at four possible frequencies, so the signal is split into a quartet.
The signals from the benzene protons have also been split as a result of magnetic non-equivalence and resulting spin-spin coupling. The question arises why the CH2 and CH3 protons of ethylbenzene interact with each other whereas the two comparable groups of protons in benzylacetate do not. The answer lies in the number of bonds separating the two groups. In ethylbenzene the two proton groups are attached to adjacent carbon atoms and may be expected to interact sufficiently with each other. In benzylacetate however, the two carbon atoms Cc and Cb are connected across two extra bonds between oxygen and another carbon atom. As a result the proton groups are too far away from each other to display significant spin-spin coupling.