Proton NMR - Chemical Shift
Since 1H is the most commonly observed isotope in NMR experiments, this shall now be dealt with in greater detail. A 1H nucleus contains a single proton and spectra in which 1H is the observe nucleus are normally referred to as proton spectra.
It was stated previously that a proton in an 11.7 T magnet will display a basic resonance frequency of approximately 500 MHz, but that the precise resonance frequency will depend upon the local atomic environment. A proton in a molecule of chloroform resonates at a slightly different frequency compared to a proton in benzene (C6H6). Therefore, the emitted frequency acts as a label which gives analysts qualitative information on the local atomic neighborhood in which a proton is located. This is the basis of NMR.
The variation in precise resonance frequency is referred to as the “chemical shift ”. The resonance frequency is shifted by the effect of neighboring atoms and in particular the extent of magnetic shielding from local electrons discussed earlier. The size of the shift is normally measured in ppm relative to the TMS peak which is referenced to 0 ppm.
Most protons, regardless of the organic compound to which they are bonded, display chemical shifts within 14 ppm of TMS.
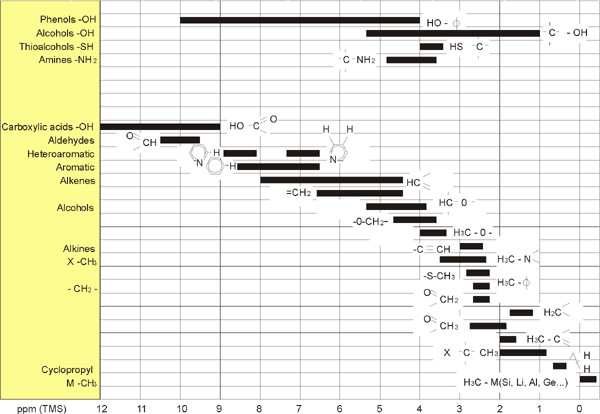
1H Chemical Shifts in Organic Compounds
The figure above is an illustration of typical proton chemical shifts in organic compounds.
| Glossary entry: | chemical shift |
The variation in precise resonance frequency.