Research Publications
Independent Publications
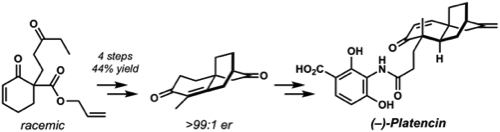
12. "Short Enantioselective Formal Synthesis of (–)-Platencin" Defeiber, C. D.; Mohr, J. T.; Grabovyi, G. A.; Stoltz, B. M. Synthesis 2018, 50, 4359–4368. [Supporting Information] (Special Issue in honor of Prof. Scott Denmark)
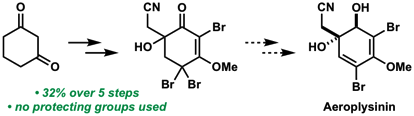
11. “Synthetic studies toward the total synthesis of aeroplysinin" Grabovyi, G. A.; Mohr, J. T. ARKIVOC 2018, part iv, 215–230. [Supporting Information] (Special Issue in honor of Prof. Gordon Gribble's retirement)
10. “Ag(II)-Mediated Synthesis of β-Fluoroketones via Oxidative Cyclopropanol Opening" Deng, Y.; Kauser, N. I.; Islam, S. M.; Mohr, J. T. Eur. J. Org. Chem. 2017, 5872–5879. [Supporting Information]

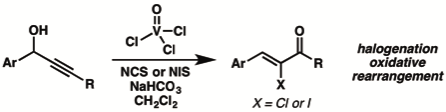
9. “Vanadium(V)-Mediated Rearrangement/Halogenation Cascade: Synthesis of α-Haloenones from Propargyl Alcohols" Zhao, M.; Mohr, J. T. Tetrahedron 2017, 73, 4115–4124. [Supporting Information] (Special Issue on New Advances in Pericyclic Reactions edited by Prof. Uttam Tambar)
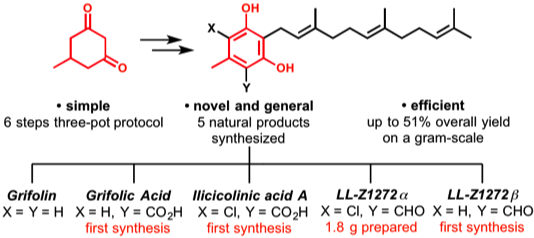
8. “Total Synthesis of Grifolin, Grifolic Acid, LL-Z1272α, LL-Z1272β, and Ilicicolinic Acid A" Grabovyi, G. A.; Mohr, J. T. Org. Lett. 2016, 18, 5010–5013. [Supporting Information] (This paper was highlighted here on organic-chemistry.org!)
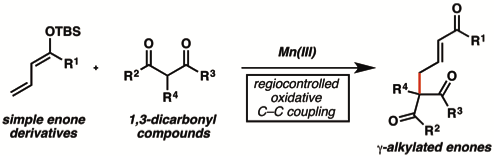
7. “Regiocontrolled Oxidative C–C Coupling of Dienol Ethers and 1,3-Dicarbonyl Compounds" Liu, X.; Chen, X.; Mohr, J. T. Org. Lett. 2016, 18, 3182–3185. [Supporting Information]
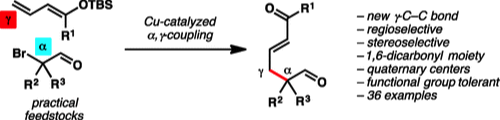
6. “Cu-Catalyzed Stereoselective γ-Alkylation of Enones" Chen, X.; Liu, X.; Mohr, J. T. J. Am. Chem. Soc. 2016, 138, 6364–6367. [Supporting Information]
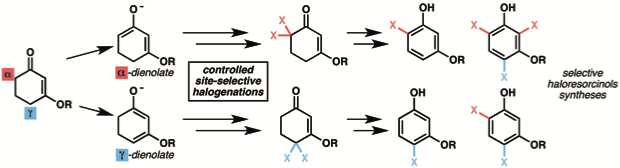
5. “Practical Regioselective Halogenation of Vinylogous Esters: Synthesis of Differentiated mono-Haloresorcinols and Polyhalogenated Resorcinols" Chen, X.; Liu, X.; Martinez, J. S.; Mohr, J. T. Tetrahedron 2016, 72, 3653–3665. [Supporting Information] (Special Issue in honor of the Tetrahedron Young Investigator Award for Prof. Neil Garg)
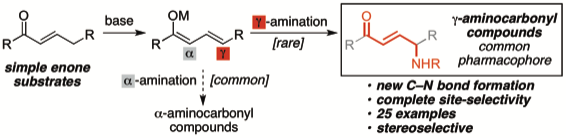
4. “Direct Regioselective γ-Amination of Enones" Chen, X.; Liu, X.; Mohr, J. T. Org. Lett. 2016, 18, 716–719. [Supporting Information]
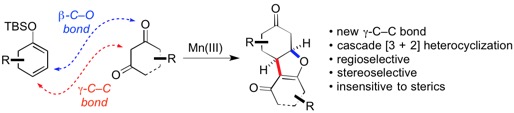
3. “Cascade Mn-mediated γ-Alkylation/oxa-Michael Addition of Enones with 1,3-Dicarbonyls" Liu, X.; Chen, X.; Mohr, J. T. Chem. – Eur. J. 2016, 22, 2274–2277. [Supporting Information]
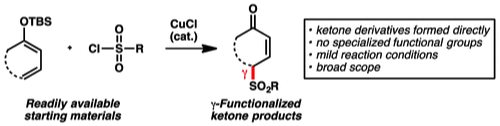
2. “Copper-Catalyzed γ-Sulfonylation of α,β-Unsaturated Carbonyl Compounds by Means of Silyl Dienol Ethers" Liu, X.; Chen, X.; Mohr, J. T. Org. Lett. 2015, 17, 3572–3575. [Supporting Information]
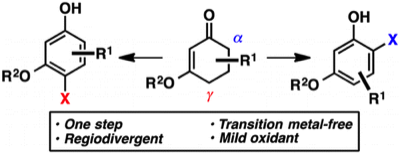
1. “Regiodivergent Halogenation of Vinylogous Esters: One-Pot, Transition Metal-Free Access to Differentiated Haloresorcinols" Chen, X.; Martinez, J. S.; Mohr, J. T. Org. Lett. 2015, 17, 378–381. [Supporting Information]
Supervised Publications
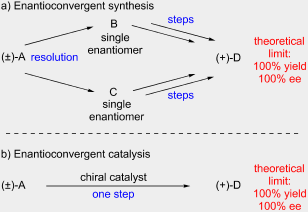
22. “Enantioconvergent Catalysis" Mohr, J. T.; Moore, J. T.; Stoltz, B. M. Beilstein J. Org. Chem. 2016, 12, 2038–2045.
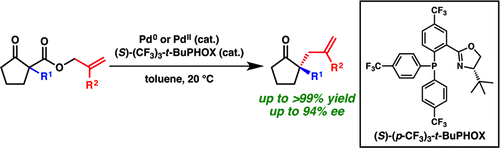
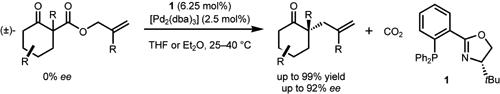
21. “Palladium-Catalyzed Enantioselective Decarboxylative Allylic Alkylation of Cyclopentanones" Craig, R. A, II; Loskot, S. A.; Mohr, J. T.; Behenna, D. C.; Harned, A. M.; Stoltz, B. M. Org. Lett. 2015, 17, 5160–5163. [Supporting Information]
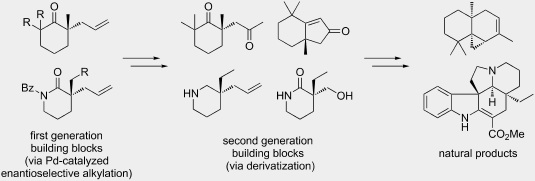
20. “Formal total syntheses of classic natural product target molecules via palladium-catalyzed enantioselective alkylation" Liu, Y.; Liniger, M.; McFadden, R. M.; Roizen, J. L.; Malette, J.; Reeves, C. M.; Behenna, D. C.; Seto, M.; Kim, J.; Mohr, J. T.; Virgil, S. C.; Stoltz, B. M. Beilstein J. Org. Chem. 2014, 10, 2501–2512. [Supporting Information]
19. “Aza-β-Lactams Compounds and Methods of Using” Cravatt, B. F.; Zuhl, A. M.; Bachovchin, D. A.; Matthews, M.; Fu, G. C.; Mohr, J. T.; Berlin, J. M. International Patent Application, WO 2013152272, 2013.
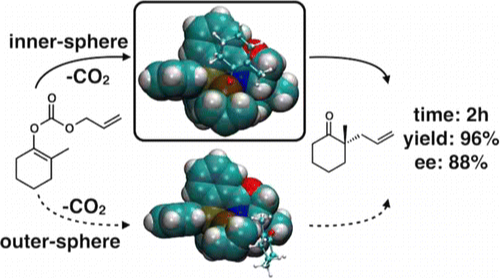
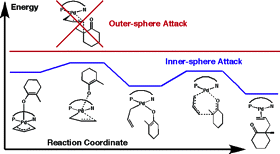
18. “The Reaction Mechanism of the Enantioselective Tsuji Allylation: Inner-Sphere and Outer-Sphere Pathways, Internal Rearrangements and Asymmetric C-C Bond Formation” Keith, J. A.; Behenna, D. C.; Sherden, N.; Mohr, J. T.; Ma, S.; Marinescu, S. C.; Nielsen, R. J.; Oxgaard, J.; Stoltz, B. M.; Goddard III, W. A. J. Am. Chem. Soc. 2012, 134, 19050–19060. [Supporting Information]
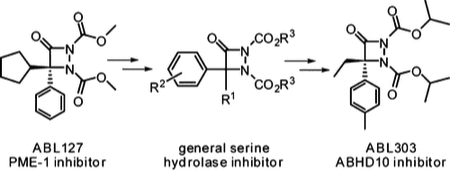
17. “Competitive Activity-Based Protein Profiling Identifies Aza-β-Lactams as a Versatile Chemotype for Serine Hydrolase Inhibition” Zuhl, A. M.; Mohr, J. T.; Bachovchin, D. A.; Niessen, S.; Hsu, K.-L.; Berlin, J. M.; Dochnahl, M.; López-Alberca, M. P.; Fu, G. C.; Cravatt, B. F. J. Am. Chem. Soc. 2012, 134, 5068–5071. [Supporting Information]
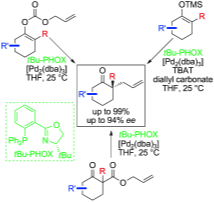
16. “Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies” Behenna, D. C.; Mohr, J. T.; Sherden, N. H.; Marinescu, S. C.; Harned, A. M.; Tani, K.; Seto, M.; Ma, S.; Novák, Z.; Krout, M. R.; McFadden, R M; Roizen, J. L.; Enquist Jr., J. A.; White, D. E.; Levine, S. R.; Petrova, K. V.; Iwashita, A.; Virgil, S. C.; Stoltz, B. M. Chem.–Eur. J. 2011, 17, 14199–14223. [Supporting Information]
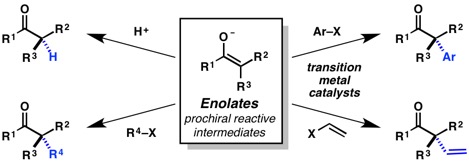
15. “Protonation, Alkylation, Arylation, and Vinylation of Enolates” Stoltz, B. M.; Mohr, J. T. In Stereoselective Synthesis, Vol. 3 (P. A. Evans, Ed.) Chapter 3.15 (2011), pp. 615–674.
14. “Academic Cross-fertilization by Public Screening Yields a Remarkable Class of Protein Phosphatase Methylesterase-1 Inhibitors” Bachovchin, D. A.; Mohr, J. T.; Speers, A. E.; Wang, C.; Berlin, J. M.; Spicer, T. P.; Fernandez-Vega, V.; Chase, P.; Hodder, P. S.; Schürer, S. C.; Nomura, D. K.; Rosen, H.; Fu, G. C.; Cravatt, B. F. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 6811–6816. [Supporting Information]
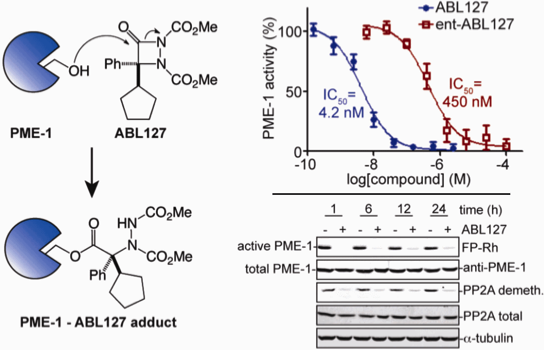
13. “Enantioselective Protonation” Mohr, J. T.; Hong, A. Y.; Stoltz, B. M. Nature Chem. 2009, 1, 359–369.
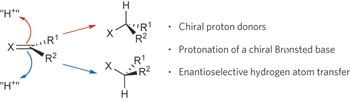
12. “Preparation of (S)-2-Allyl-2-methylcyclohexanone” Mohr, J. T.; Krout, M. R.; Stoltz, B. M. Org. Synth. 2009, 86, 194–211.
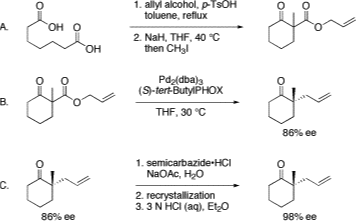
11. “Preparation of (S)-tert-ButylPHOX” Krout, M. R.; Mohr, J. T.; Stoltz, B. M. Org. Synth. 2009, 86, 181–193.
10. “Enantioselective Total Synthesis of (+)-Cassiol” Petrova, K. V.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2009, 11, 293–295. [Supporting Information]

9. “Natural Products as Inspiration for Development of Asymmetric Catalysts” Mohr, J. T.; Krout, M. R.; Stoltz, B. M. Nature 2008, 455, 323–332.
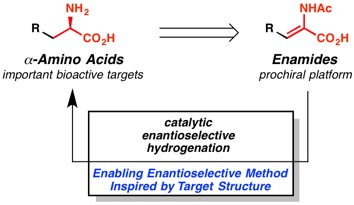
8. “Homogeneous Pd-Catalyzed Enantioselective Decarboxylative Protonation” Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039–1042. [Supporting Information]
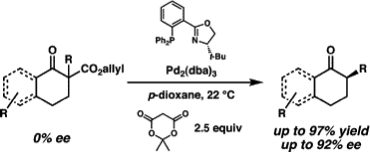
7. “Enantioselective Tsuji Allylations” Mohr, J. T.; Stoltz, B. M. Chem.–Asian J. 2007, 2, 1476–1491.
6. “Catalytic Enantioselective Stereoablative Reactions: An Unexploited Approach to Enantioselective Catalysis” Mohr, J. T.; Ebner, D. C.; Stoltz, B. M. Org. Biomol. Chem. 2007, 5, 3571–3576.
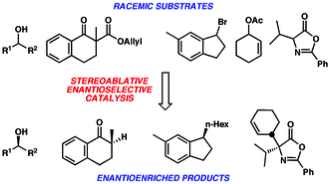
5. “The Inner-Sphere Process in the Enantioselective Tsuji Allylation Reaction with (S)-t-Bu-phosphinooxazoline (PHOX) Ligands” Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348–11349. [Supporting Information]
4. “Process for Enantioselective Allylation of Ketones and Olefins” Behenna, D. C.; Stoltz, B. M.; Mohr, J. T.; Harned, A. M. United States Patent, US 7235698, 2007.
3. “Catalytic Enantioselective Decarboxylative Protonation” Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348–11349. [Supporting Information]
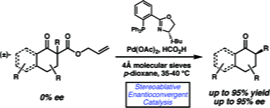
2. "Deracemization of Quaternary Carbon Stereocenters by Pd-Catalyzed Enantioconvergent Decarboxylative Allylation of Racemic β-Ketoesters" Mohr, J. T.; Behenna, D. C.; Harned, A. M.; Stoltz, B. M. Angew. Chem., Int. Ed. 2005, 44, 6924–6927. [Supporting Information]
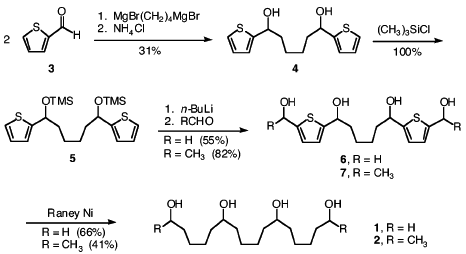
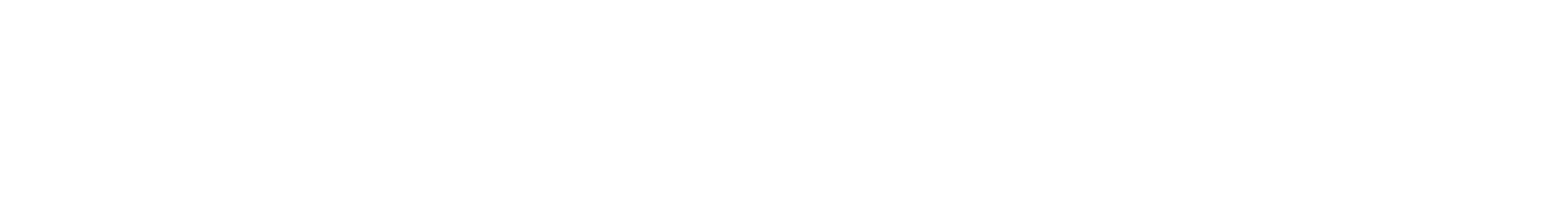
1. "Anesthetic Potency of Two Novel Synthetic Polyhydric Alkanols Longer than the n-Alkanol Cutoff: Evidence for a Bilayer-Mediated Mechanism of Anesthesia?" Mohr, J. T.; Gribble, G. W.; Lin, S. S.; Eckenhoff, R. G.; Cantor, R. S. J. Med. Chem. 2005, 48, 4172–4176.