
|
|
|
Our Research:
We develop and apply chemical methods to label proteins and study their function in living mammalian cells. With chemical labeling, a protein of interest is expressed as a fusion to a polypeptide tag that interacts specifically with a synthetic fluorophore or other chemical probe (Figure 1). Synthetic fluorophores can offer distinct advantages over commonly used fluorescent protein labels, such as superior brightness, longer fluorescence lifetime,
higher photostability or red-shifted emission. Chemical probes can also imbue proteins with properties that are not easily encoded genetically, for example environmental sensitivity, photo-crosslinking ability or high electron density. Our experimental approach incorporates synthetic organic chemistry, molecular and cell biology, and microscopy.
Our current research interests include:
|
| |
1) Multiplexed chemical labeling of proteins in living cells: Molecules that bind specifically and with high affinity to proteins can be developed into powerful tools for chemical biology. The interaction between substituted 5-benzyl pyrimidines and different forms of dihydrofolate reductase (DHFR) can be exploited for chemically labeling fusion proteins in mammalian cells. For example, the specific, high affinity (KD ~ 1 nM) interaction between
trimethoprim and E. coli DHFR has been exploited to develop the LigandLink(tm) Universal Labeling technology (Active Motif, Inc., Carlsbad, CA, www.activemotif.com) that makes it possible to tag eDHFR fusion proteins in wild-type mammalian cells with cell-permeable trimethoprim-fluorophore conjugates (Figure 1). We are currently developing additional antifolate/DHFR pairs for in vivo protein labeling, with the ultimate goal being the simultaneously labeling of multiple proteins in a single cell.
2) Time-resolved microscopy of lanthanide probes. Lanthanide luminescence offers several advantages for fluorescence-based biological assays: 1) large Stoke’s shifts (>150 nm) and multiple, narrow emission bands (<10 nm at half-maximum) allow efficient spectral separation of emission signals; 2) long luminescence lifetimes (micro- to millisecond) enable time-resolved detection methods to remove scattering and autofluorescence background; and 3) relative insensitivity to photobleaching
allows for prolonged detection. We are exploring time-resolve microscopy (Figure 2) as a means of imaging lanthanide probe-labeled proteins at the single-molecule limit of detection.
3) Dynamic visualization of protein-protein interactions: Transient protein-protein interactions control cell growth and function. We are developing lanthanide-based, protein labels with long (msec) luminescent lifetimes (Figure 2). These probes will facilitate in vivo, time-resolved imaging of resonance energy transfer between
interacting proteins. By analyzing the luminescent decay curves of long-lifetime donors, it will be possible to determine the stoichiometry of the interaction, as well as the dynamic localization within the cell. Furthermore, it will be possible to time-gate out any background fluorescence from endogenous biomolecules, allowing luminescent live cell imaging with unprecedented signal-to-noise ratio.
|
| |
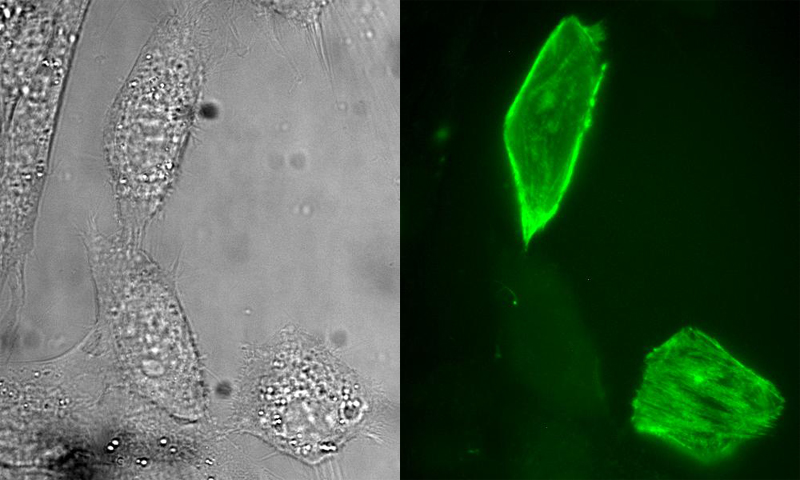 |
Figure 1. Selective labeling of a fusion protein with a small molecule in living cells. Epi-fluorescence micrographs (left, bright field; right, fluorescence) of NIH3T3 cells that were transiently transfected with DNA encoding a fusion of myosin light chain (MLC) to E. coli dihydrofolate reductase (eDHFR). The cells were incubated in growth medium containing a heterodimeric conjugate of trimethoprim (TMP)
|
| |
covalently linked to a fluorescein derivative. The TMP-fluorescein diffuses into cells and binds selectively and non-covalently to the MLC-eDHFR fusion protein which is associated with actin stress fibers.
|
| |
|
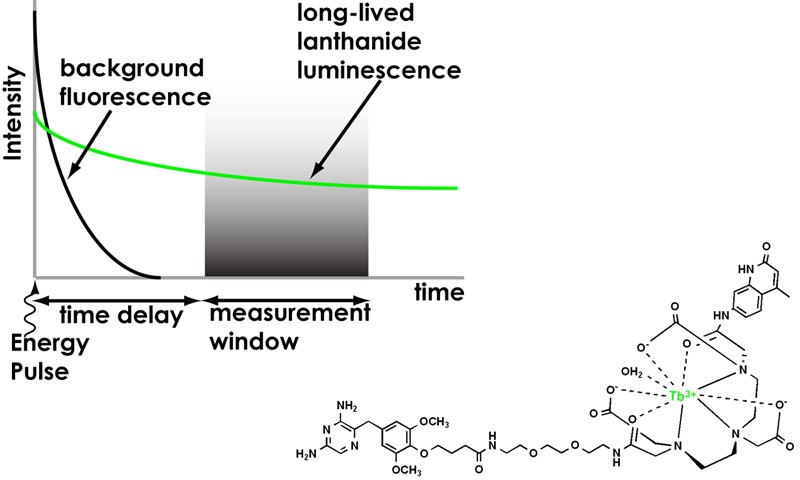
Figure 2. (upper left) Principle of time-resolved detection. Short pulses of illumination excite samples. Lanthanide probe molecules with long luminescent lifetimes (millisecond) are detected after a brief (microsecond) delay, eliminating short-lifetime scattering and autofluorescence background signals. We have developed a time-resolved epi-fluorescence microscope to image lanthanide luminescence in living cells at high signal-to-background ratio.
|
| |
(lower right) A luminescent terbium protein label. Probes of this type selectively impart lanthanide luminescence to fusion proteins, allowing time-resolved imaging and detection in vitro and in living cells.
|
|

Address:Room 4500, Science & Engineering South (SES), 845
W Taylor St, Chicago, Illinois,USA. 60607
Email:
lwm2006@uic.edu
|

