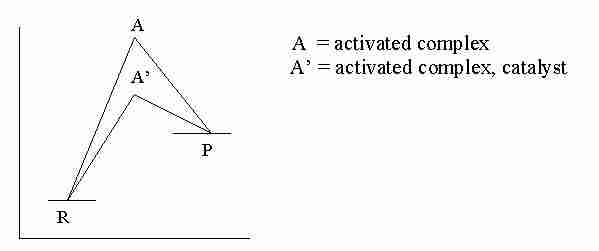
A. Concept.
|
rate =
|
| Usually expressed in terms of reactant | : |
Rate:
Because it is a reactant a minus sign is used to make rate a positive quantity.
Concentration will be expressed in moles/liter; time may be in seconds, minutes, years. . . .
B. Dependence on concentration of reactant(s)
| Single Reactant | Rate: k(conc. Reactant)m; k = rate constant, m = order |
| Two Reactants | Rate = k(conc. Reactant 1)m
(conc. Reactant 2)n Overall order = m + n |
Generally, m and n are positive integers (1,2,3). However, they can be
0 or a fraction such as ½.
See overhead #3
Determination of m and k from rate-concentration data:
| CH3CHO(g) |
CH4(g) |
CO(g) | |
| Rate | 2.0 M/s | 0.50 M/s | 0.080 M/s |
| Conc. CH3CHO | 1.0 M | 0.50 M | 0.2 M |
rate 1/ rate 2 = (conc. 1/conc. 2)m; ** KNOW! Very important!
2.0 / 0.50 = (1.0 /
0.50)m ![]() m = 2 Second
Order
m = 2 Second
Order
hence, rate = k(conc. CH3CHO)2
k = rate (conc. CH3CHO)2 = (2.0 M/s) /
(1.0 M)2 = 2.0 M-1s-1,
See Overhead #4 See Figure 18.1 p.617
You CAN'T look at the balanced equation and write a rate expression.
It must be done experimentally. (see top of p. 617). Table 18.1
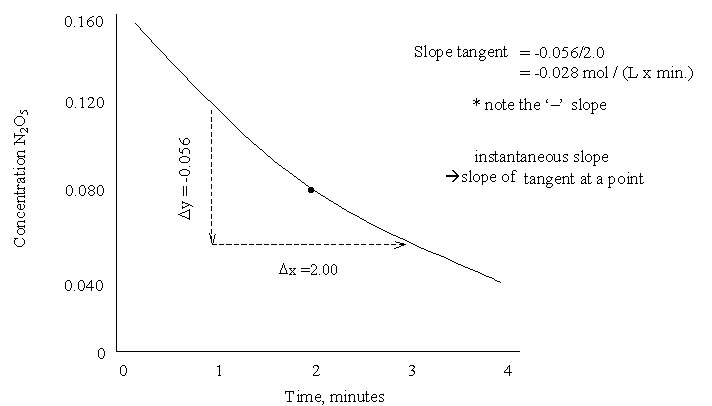
2N2O5 ![]() 4 NO2
4 NO2 ![]() O2
O2
Rate = -![]() conc./
conc./
![]() time = -
time = -![]() [N2O5] /
[N2O5] / ![]() t
t
Rate = k [N2O5]1
1st order
k = Rate Constant
Rate = k (conc. N2O5)
k = a rate "constant". . .but it does depend
on temp.!
here m = 1 so 1st order
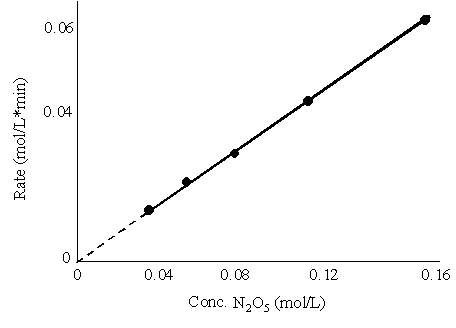
Figure 18.2, p. 619
You need to be able to look at data like this and write a rate expression:
Do Separate
Rate 2/Rate 1 = ([conc. 2]/[conc. 1])m
Table 18.2 Initial Rates of Reaction (mol/L ·
s) at 55°C for
|
|||||||||||||||||
| (CH3)3CBr | OH- | (CH3)3CBr | OH- | ||||||||||||||
|
(mol/L)
|
(mol/L)
|
RATE
|
(mol/L) |
(mol/L)
|
RATE
|
||||||||||||
|
Expt. 1 |
0.50 |
0.050 |
0.0050 |
1.0 |
0.050 |
0.010 |
|||||||||||
|
Expt. 2 |
1.0 |
0.050 |
0.010 |
1.0 |
0.10 |
0.010 |
|||||||||||
|
Expt. 3 |
1.5 |
0.050 |
0.015 |
1.0 |
0.15 |
0.010 |
|||||||||||
|
Expt. 4 |
2.0 |
0.050 |
0.20 |
1.0 |
0.20 |
0.010 |
|||||||||||
Rate = k(conc. (CH3)3CBr)m(conc. OH-)n
Rate = k(conc. (CH3)3CBr)(conc. OH-)0 = k(conc. (CH3)3CBr)
C. First Order Reaction
ln X0/X = kt; X0 = original conc., X = conc. at time 't'
Suppose k = 0.250s-1, X0 = 1.00 M. What is the concentration of reactant after 10.0 s?
HALF LIFE
-t1/2 is independent of original concentration.
It takes as long for conc. to drop from 1.0M to 0.50M as it takes-t1/2 is inversely related to 'k'. If t1/2 is small, 'k' is large and vice versa.
to drop from 2.0M to 1.0M.So
ln X0/X = kt1/2 = ln 1/ (1/2) = ln 2. . . see overhead #6
ln X0 - ln X = kt or ln X = -kt + ln X0, which takes this formy = mx +b
See Overhead #7
Rate = -dx/dt for 1st order, rate = kx
So kx = -dx/dt or dx/x = -k· dt
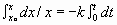
KNOW!
¯
ln X – ln X0 =
-kt OR ln X0 – ln X =
kt Þ

ln x = -kt + ln X0
-k = slope, ln X0 = y-intercept
Figure 18.3, p. 625à For a first-order reaction, a plot of
ln conc. vs time is a straight line.