


|
Effects of temperature,
concentration, catalysts, inhibitors on reaction
rates |
|
After studying this lesson you should be able
to: |
- describe how temperature changes alter reaction
rates
- explain what makes for "effective"
collisions
- explain what results in "ineffective"
collisions
- describe how reactant concentrations influence
the initial rate of a reaction
- describe how temperature changes influence the
initial rate of a reaction
- tell what effect a catalyst has on the initial
rate of a reaction
- describe the action of a catalyst on a reaction
pathway, give one example
- give definitions for effective collisions,
ineffective collisions, catalyst, inhibitor, reaction rate
|
|
Collision theory and temperature effects
on rates |
|
Kinetic theory says that molecules are in constant
motion. The kinetic energy and molecule velocity increase with
temperature. KE =
[1/2][mv2] |
|
Reactions usually require collisions between
reactant molecules or atoms. The formation of bonds requires atoms to come
close to one another. New bonds can form only if the atoms are close
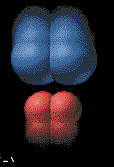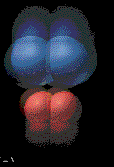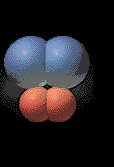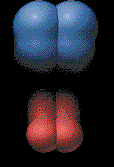
enough together to share electrons. Some collisions are not successful.
These are called ineffective
collisions. The particles simply hit and
then rebound. This animation illustrates what happens in an ineffective
collision. |
|
|
Collisions that lead to products are called
effective collisions. An effective collision must
happen with a great enough speed, energy and force to break bonds in the
colliding molecules.
The animation illustrates an effective collision between two diatomic molecules. The two product
molecules formed fly outwards. |
|
|
Collisions between molecules will be more violent
at higher temperatures. The higher temperatures mean higher velocities.
This means there will be less time between collisions. The frequency of
collisions will increase. The increased number of collisions and the
greater violence of collisions results in more effective collisions. The
rate for the reaction increases. Reaction rates are roughly doubled when
the temperature increases by 10 degrees Kelvin. This
means the rate can be quadrupled if the temperature is raised by 20
degrees Kelvin. |
|
It should be clear that if you can increase
reaction rates by increasing temperature you can decrease reaction rates
by lowering the temperature. You do this every time you put something in
the refrigerator. If you want to see the effect of elevated temperatures
increased reaction rates you can leave some dairy product out of the
refrigerator for a few days and compare its condition with the same age
dairy product that was kept cold. |
|
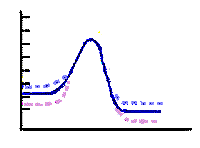
Temperature effects on rates and
activation energy diagram |
- This illustration shows what happens to an
exothermic reaction when the temperature is changed.
- The dotted blue curve
shows the energy for a reaction mixture
that is heated. The reactants are "part way" up the energy barrier
because they are "hot".
- The dotted magenta curve shows
what cooling does to the reactant energy. The energy goes down and the
reaction happens with more difficulty.
-
- NOTE: The energies of reactants and
products have changed. They both have different energies because they
were either heated or cooled. The heat of reaction is the slightly
different. The relative amounts of reactants and products are slightly
different because of the temperature changes.
|
|
|
Reaction rates can be increased if the
concentration of reactants is raised. An increase in concentration
produces more collisions. The chances of an effective collision goes up
with the increase in concentration. The exact relationship between
reaction rate and concentration depends on the reaction "mechanism".
This is the process involving elementary reaction steps. The slowest
step controls the rate. The nature of the slow step is not obvious from
the balanced equation. Only experimental observation reveals the link
between concentration and reaction
rates. |
|
The reaction energy path controls the speed of
the reaction. The molecules follow the path of least resistance, but
this path may still require a lot of energy. The activation energy for
the path may be high and then the reaction will be
slow. |
|
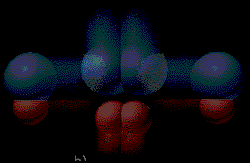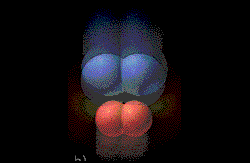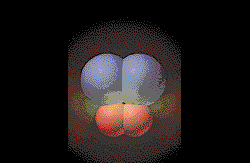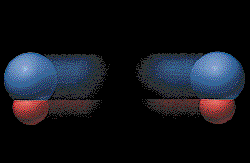
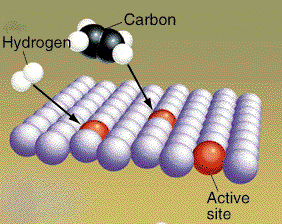
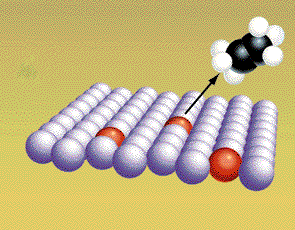
A reaction pathway can be altered by adding
nonreacting compounds to the reaction mixture. These molecules can
sometimes alter the pathway so the energy needed for reaction is
lowered. When this happens the reaction rates are faster. A material
that lowers the activation energy is called a catalyst. The four
pictures show the effect of a catalyst on hydrogenation of ethylene. The
CH2CH2 and H2 molecules
are attracted to the metal catalyst.
(Graphic by permission of Prentice Hall)
.
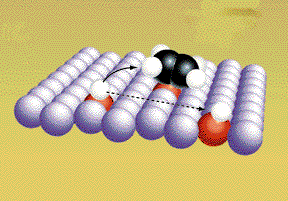
The covalent bonds are weakened because the metal
atoms attract electrons away form the "bonded" atoms. The bonds break.
(Graphic by permission of Prentice
Hall)
|
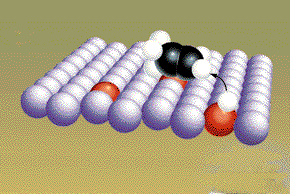
The free radicals from the original
molecules move along the surface of the metal until they collide
and form products. (Graphic by
permission of Prentice
Hall) |
|
The ethane, CH3CH3 is
not as "electron rich" as the ethylene, so it is not attracted
strongly to the metal. The ethane breaks away from the catalyst.
The metal catalyst is not consumed in the reaction, but the
mechanical action of flowing gases wears or erodes the metal away
and the catalyst must be replenished. (Graphic by permission of Prentice
Hall) |
The catalyst is not consumed in the reaction. It
merely speeds up the reaction by providing a lower energy path between
reactants and products. Catalysts associate with reactant molecules (in
biochemistry the reactant molecules are called substrates) and cause a
redistribution of electron densities in the reacting molecules
(substrates). The bonds that a need to be broken in the reaction are
weakened by the association with the catalyst. This makes the reaction
occur faster because the weakened bonds are easier to break. When the
substrate reacts the catalyst molecule is released and able to repeat
the process with another reactant substrate molecule. Enzymes are
biological catalysts. Enzymes have a big molecular size. The enzyme is
usually very large compared to the reacting substrate. Enzymes have
folds and creases and the reactant molecules fit into a definite
location in these folds and creases. The substrate molecule ties onto
the enzyme molecule at a these "active sites" . The term "lock and key"
is applied to this interaction. The enzyme is the "lock" and the
substrate is the "key". Problems occur in biological systems when the
catalyst is poisoned. This can happen when the active site is "picked"
or tied up by a molecule. This kind of thing sometimes happens when
heavy metals react with enzymes. One reason why heavy metals are toxic
is that they bind to important metabolic enzymes. This keeps the enzyme
from carrying out its normal function and the organism
suffers. |
|
Inhibitors are the exact opposite of catalysts.
They increase the energy of the reaction pathway. In fact they often
block the normal pathway. This is desirable when you want to preserve
molecules and slow decomposition
reactions. |






