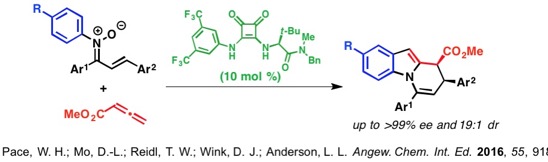
Heterocyclic molecules are commonly found in both medicinal and material applications. Due to continuously increasing demands for more efficient routes to known heterocycles and discoveries of new heterocycles, the design of innovative methods that facilitate access to these important scaffolds are required.
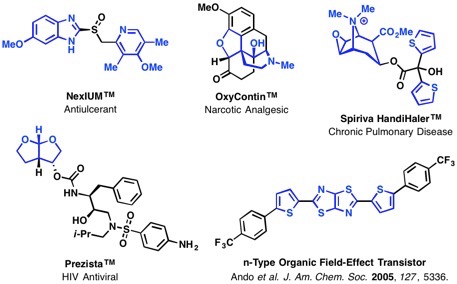
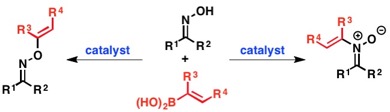
To streamline the synthesis of heterocyclic and heteroatom-functionalized molecules, our laboratory has pursued the development of novel transformations that employ oximes and hydroxamic acids. We have discovered conditions to unlock and control the reactivity embedded in the N–O bond to achieve a diverse array of new reactions and to efficiently prepare challenging C–O, C–N, and C–C bonds from simple starting materials. The diagram below illustrates our efforts focused on accessing and harnessing the rearrangement reactivity of O-vinyl- and O-aryl hydroxylamines, as well as N-aryl- or N-vinylnitrones. We have developed simple methods for generating both types of rearrangement precursors from hydroxylamines and oximes by treatment with aryl- or vinylboronic acids under Chan-Lam-Evans conditions that selectively give either C–O or C–N bond coupling products based on the structure of the hydroxylamine or oxime starting material. We have then used these compounds to trigger new rearrangements and facilitate rapid access to important synthons and heterocyclic motifs.
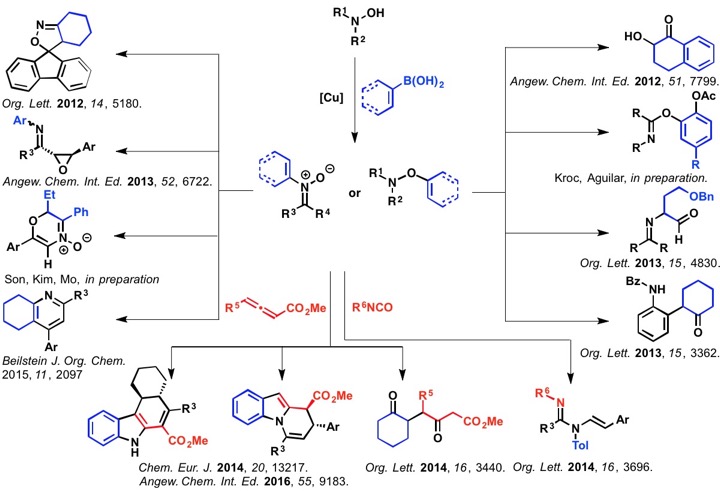
Our current research efforts are focused in several directions:
1. Expanding our repertoire of new rearrangement reactions of hydroxylamines, oximes, and nitrones for the synthesis of new and challenging heterocyclic compounds.
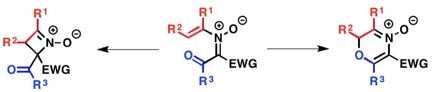
2. Advancing our methods by creating asymmetric variants.
3. Studying the mechanism of the chemoselectivity of the C–N, and C–O bond forming processes discovered in our laboratory.
4. Using high-throughput experimentation techniques to facilitate and expedite new method development.

5. Applying our methods to medicinal chemistry applications with collaborations established through the UICentre for Drug Discovery.




If you are an undergraduate student considering research opportunities in synthetic chemistry or a first-year graduate student considering joining a synthetic organic research lab, check out the link below to a short movie about day-to-day research activities in our lab. You must by logged into Google Drive with a UIC email address to access this content. link
