Publications
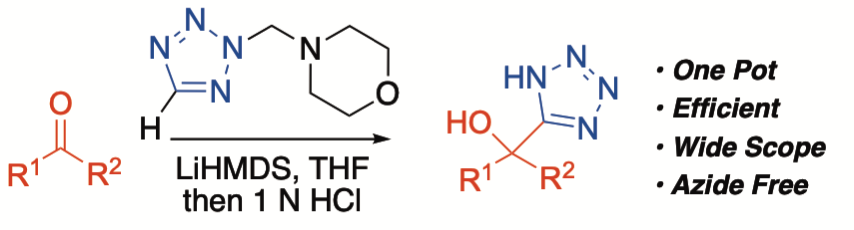
N-Morpholinomethyl-5-lithiotetrazole: A Reagent for the One-Pot Synthesis of 5-(1-Hydroxyalkyl) tetrazoles
Panagiotis D. Alexakos and Duncan J. Wardrop
J. Org. Chem. 2019, 84, 12430-12436
DOI: 10.1021/acsmedchemlett.7b00313.
[Supporting Information]
Panagiotis D. Alexakos and Duncan J. Wardrop
J. Org. Chem. 2019, 84, 12430-12436
DOI: 10.1021/acsmedchemlett.7b00313.
[Supporting Information]
Abstract
An efficient, one-pot method for the preparation of 5-(1-hydroxyalkyl)tetrazoles is reported. N-Morpholinomethyl-5-lithiotetrazole, generated by the deprotonation of 4-(N-tetrazolylmethyl)morpholine with LiHMDS, undergoes addition to ketones and aldehydes (both aromatic and aliphatic) to form 5-(1-hydroxyalkyl)tetrazoles in a high yield, after acidic workup. The reported protocol displays a broad substrate scope and functional group tolerance, avoids the use of cyanide- or azide-based reagents, and provides access to sterically congested and unsaturated tetrazoles, which are difficult to access by other means.
An efficient, one-pot method for the preparation of 5-(1-hydroxyalkyl)tetrazoles is reported. N-Morpholinomethyl-5-lithiotetrazole, generated by the deprotonation of 4-(N-tetrazolylmethyl)morpholine with LiHMDS, undergoes addition to ketones and aldehydes (both aromatic and aliphatic) to form 5-(1-hydroxyalkyl)tetrazoles in a high yield, after acidic workup. The reported protocol displays a broad substrate scope and functional group tolerance, avoids the use of cyanide- or azide-based reagents, and provides access to sterically congested and unsaturated tetrazoles, which are difficult to access by other means.
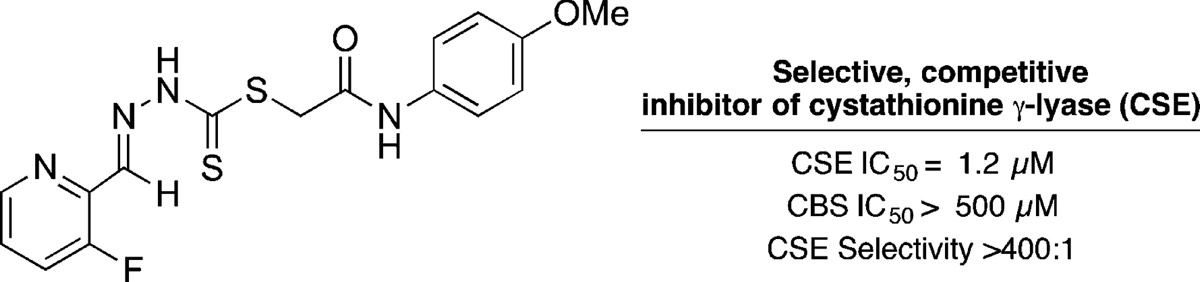
2-Arylidene Hydrazinecarbodithioates as Potent, Selective Inhibitors of Cystathionine γ-Lyase (CSE)
Abir Bhattacharjee, Antara Sinha, Kiira Ratia, Liang Yin, Loruhama Delgado-Rivera, Pavel A Petukhov
Gregory R. J. Thatcher, and Duncan J. Wardrop
ACS Med. Chem. Lett. 2017, 8, 1241–12451241–1245
DOI: 10.1021/acsmedchemlett.7b00313
[Supporting Information]
Abir Bhattacharjee, Antara Sinha, Kiira Ratia, Liang Yin, Loruhama Delgado-Rivera, Pavel A Petukhov
Gregory R. J. Thatcher, and Duncan J. Wardrop
ACS Med. Chem. Lett. 2017, 8, 1241–12451241–1245
DOI: 10.1021/acsmedchemlett.7b00313
[Supporting Information]
Abstract
Hydrogen sulfide is produced from l-cysteine by the action of both cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) and increasingly has been found to play a profound regulatory role in a range of physiological processes. Mounting evidence suggests that upregulation of hydrogen sulfide biosynthesis occurs in several disease states, including rheumatoid arthritis, hypertension, ischemic injury, and sleep-disordered breathing. In addition to being critical tools in our understanding of hydrogen sulfide biology, inhibitors of CSE hold therapeutic potential for the treatment of diseases in which increased levels of this gasotransmitter play a role. We describe the discovery and development of a novel series of potent CSE inhibitors that show increased activity over the benchmark inhibitor and, importantly, display high selectivity for CSE versus CBS.
Hydrogen sulfide is produced from l-cysteine by the action of both cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) and increasingly has been found to play a profound regulatory role in a range of physiological processes. Mounting evidence suggests that upregulation of hydrogen sulfide biosynthesis occurs in several disease states, including rheumatoid arthritis, hypertension, ischemic injury, and sleep-disordered breathing. In addition to being critical tools in our understanding of hydrogen sulfide biology, inhibitors of CSE hold therapeutic potential for the treatment of diseases in which increased levels of this gasotransmitter play a role. We describe the discovery and development of a novel series of potent CSE inhibitors that show increased activity over the benchmark inhibitor and, importantly, display high selectivity for CSE versus CBS.
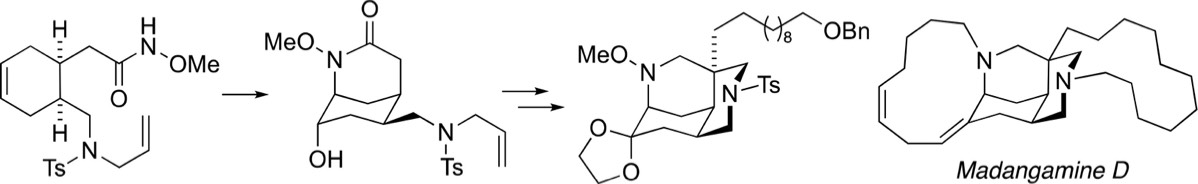
Oxamidation of Unsaturated O-Alkyl Hydroxamates: Synthesis of the Madangamine Diazatricylic (ABC Rings) Skeleton
Abir Bhattacharjee, Mikhail V. Gerasimov, Sam DeJong, and Duncan J. Wardrop
Gregory R. J. Thatcher, and Duncan J. Wardrop
Org. Lett. 2017, 19, 6570–6573
DOI: 10.1021/acs.orglett.7b03283
[Supporting Information]
Abir Bhattacharjee, Mikhail V. Gerasimov, Sam DeJong, and Duncan J. Wardrop
Gregory R. J. Thatcher, and Duncan J. Wardrop
Org. Lett. 2017, 19, 6570–6573
DOI: 10.1021/acs.orglett.7b03283
[Supporting Information]
Abstract
A novel approach to the diazatricyclic madangamine ABC ring system and the synthesis of an advanced, differentially protected intermediate for the synthesis of madangamine D is reported. Central to the success of this approach is the iodine(III)-mediated intramolecular oxamidation of an unsaturated O-methyl hydroxamate, a π–N+-type cyclization which proceeds in high yield and with complete regioselectivity to generate the 2-azabicyclo[3.3.1]nonane (morphan) system encompassing rings A and C.
A novel approach to the diazatricyclic madangamine ABC ring system and the synthesis of an advanced, differentially protected intermediate for the synthesis of madangamine D is reported. Central to the success of this approach is the iodine(III)-mediated intramolecular oxamidation of an unsaturated O-methyl hydroxamate, a π–N+-type cyclization which proceeds in high yield and with complete regioselectivity to generate the 2-azabicyclo[3.3.1]nonane (morphan) system encompassing rings A and C.
Inhibition of Influenza H7 Hemagglutinin-Mediated Entry
Aleksandar Antanasijevic, Nicholas J. Hafeman, Smanla Tundup, Carolyn Kingsley,
Rama K. Mishra, Lijun Rong, Balaji Manicassamy, Duncan Wardrop, and Michael Caffrey
ACS Infect. Dis., 2016, 2, 608–615
DOI: 10.1021/acsinfecdis.6b00046
Aleksandar Antanasijevic, Nicholas J. Hafeman, Smanla Tundup, Carolyn Kingsley,
Rama K. Mishra, Lijun Rong, Balaji Manicassamy, Duncan Wardrop, and Michael Caffrey
ACS Infect. Dis., 2016, 2, 608–615
DOI: 10.1021/acsinfecdis.6b00046
Abstract
The viral envelope protein hemagglutinin (HA) plays a critical role in influenza entry and thus is an attractive target for novel therapeutics. The small molecule tert-butylhydroquinone (TBHQ) has previously been shown to bind to HA and inhibit HA-mediated entry with low micromolar potency. However, enthusiasm for the use of TBHQ has diminished due to the compound’s antioxidant properties. In this work we show that the antioxidant properties of TBHQ are not responsible for the inhibition of HA-mediated entry. In addition, we have performed a structure–activity relationship (SAR) analysis of TBHQ derivatives. We find that the most promising compound, 3-tert-butyl-4-methoxyphenol, exhibits enhanced potency (IC50 = 0.6 μM), decreased toxicity (CC50 = 340 μM), and increased stability (t1/2 > 48 h). Finally, we have characterized the binding properties of 3-tert-butyl-4-methoxyphenol using NMR and molecular dynamics to guide future efforts for chemical optimization.
The viral envelope protein hemagglutinin (HA) plays a critical role in influenza entry and thus is an attractive target for novel therapeutics. The small molecule tert-butylhydroquinone (TBHQ) has previously been shown to bind to HA and inhibit HA-mediated entry with low micromolar potency. However, enthusiasm for the use of TBHQ has diminished due to the compound’s antioxidant properties. In this work we show that the antioxidant properties of TBHQ are not responsible for the inhibition of HA-mediated entry. In addition, we have performed a structure–activity relationship (SAR) analysis of TBHQ derivatives. We find that the most promising compound, 3-tert-butyl-4-methoxyphenol, exhibits enhanced potency (IC50 = 0.6 μM), decreased toxicity (CC50 = 340 μM), and increased stability (t1/2 > 48 h). Finally, we have characterized the binding properties of 3-tert-butyl-4-methoxyphenol using NMR and molecular dynamics to guide future efforts for chemical optimization.
Synthetic Applications of Nitrenium Ions
Duncan J. Wardrop and Edward G. Bowen
Nitrenes and Nitrenium Ions (eds D. E. Falvey and A. D. Gudmundsdottir),
John Wiley & Sons, Inc., Hoboken, NJ, USA
DOI: https://doi.org/10.1371/journal.pone.0076363
Duncan J. Wardrop and Edward G. Bowen
Nitrenes and Nitrenium Ions (eds D. E. Falvey and A. D. Gudmundsdottir),
John Wiley & Sons, Inc., Hoboken, NJ, USA
DOI: https://doi.org/10.1371/journal.pone.0076363
Inhibition of Influenza H7 Hemagglutinin-Mediated Entry
Aleksandar Antanasijevic, Han Cheng, Duncan J. Wardrop, Lijun Rong, and Michael Caffrey
PLoS ONE 8(10): e76363
DOI: 10.1371/journal.pone.0076363
Aleksandar Antanasijevic, Han Cheng, Duncan J. Wardrop, Lijun Rong, and Michael Caffrey
PLoS ONE 8(10): e76363
DOI: 10.1371/journal.pone.0076363
Abstract
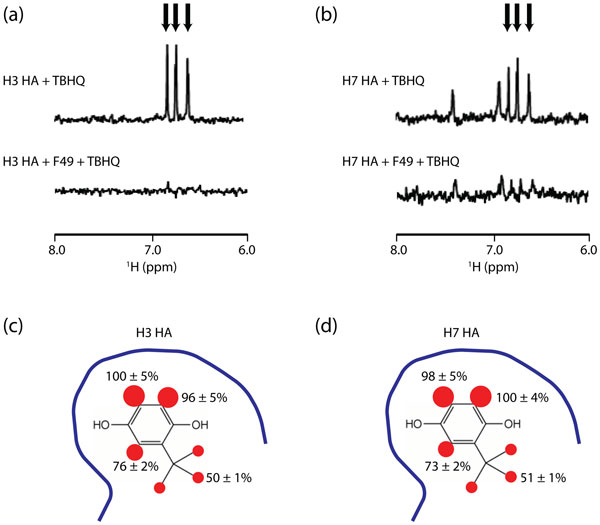
The recent outbreak of H7N9 influenza in China is of high concern to public health. H7 hemagglutinin (HA) plays a critical role in influenza entry and thus HA presents an attractive target for antivirals. Previous studies have suggested that the small molecule tert-butyl hydroquinone (TBHQ) inhibits the entry of influenza H3 HA by binding to the stem loop of HA and stabilizing the neutral pH conformation of HA, thereby disrupting the membrane fusion step. Based on amino acid sequence, structure and immunogenicity, H7 is a related Group 2 HA. In this work we show, using a pseudovirus entry assay, that TBHQ inhibits H7 HA-mediated entry, as well as H3 HA-mediated entry, with an IC50∼6 µM. Using NMR, we show that TBHQ binds to the H7 stem loop region. STD NMR experiments indicate that the aromatic ring of TBHQ makes extensive contact with the H7 HA surface. Limited proteolysis experiments indicate that TBHQ inhibits influenza entry by stabilizing the H7 HA neutral pH conformation. Together, this work suggests that the stem loop region of H7 HA is an attractive target for therapeutic intervention and that TBHQ, which is a widely used food preservative, is a promising lead compound.
The recent outbreak of H7N9 influenza in China is of high concern to public health. H7 hemagglutinin (HA) plays a critical role in influenza entry and thus HA presents an attractive target for antivirals. Previous studies have suggested that the small molecule tert-butyl hydroquinone (TBHQ) inhibits the entry of influenza H3 HA by binding to the stem loop of HA and stabilizing the neutral pH conformation of HA, thereby disrupting the membrane fusion step. Based on amino acid sequence, structure and immunogenicity, H7 is a related Group 2 HA. In this work we show, using a pseudovirus entry assay, that TBHQ inhibits H7 HA-mediated entry, as well as H3 HA-mediated entry, with an IC50∼6 µM. Using NMR, we show that TBHQ binds to the H7 stem loop region. STD NMR experiments indicate that the aromatic ring of TBHQ makes extensive contact with the H7 HA surface. Limited proteolysis experiments indicate that TBHQ inhibits influenza entry by stabilizing the H7 HA neutral pH conformation. Together, this work suggests that the stem loop region of H7 HA is an attractive target for therapeutic intervention and that TBHQ, which is a widely used food preservative, is a promising lead compound.
Iodine(III)-Mediated Cyclization of Unsaturated O-Alkyl Hydroxamates: Silyl-Assisted Access to α-Vinyl and α-(2-Silylvinyl) Lactams
Duncan J. Wardrop, Maria Yermolina, and Edward G. Bowen
Synthesis, 2012, 44, 1199-1207
DOI: 10.1055/s-0031-1290750
[Supporting Information]
Duncan J. Wardrop, Maria Yermolina, and Edward G. Bowen
Synthesis, 2012, 44, 1199-1207
DOI: 10.1055/s-0031-1290750
[Supporting Information]
Abstract
The embodiment of lactam rings within a wealth of physiologically active natural products and pharmaceutical agents ensures that the development of synthetic methods, which facilitate the preparation of these saturated N-heterocycles, is of critical importance. Herein the development of a versatile method for the synthesis of 4 to 8-membered α-vinyl and α-(2-silylvinyl) lactams involving the iodine(III)-mediated oxidative cyclization of unsaturated O-alkyl hydroxamates, which encompass an allylsilane, is reported. Importantly, the outcome of this transformation can be effectively controlled through variation of the substitution pattern at the silicon center. While allyltrimethylsilanes undergo ring closure with desilylation to form α-vinyl lactams, the corresponding triisopropyl and triphenylsilanes cyclize without loss of the larger silyl group to form E-vinylsilanes with excellent stereoselectivity. From a mechanistic standpoint, it is proposed that this reaction proceeds via concerted alkene addition of a singlet nitrenium ion (or its equivalent) to form a bicyclic N-acyl-N-alkoxyaziridinium ion, which undergoes eliminative ring opening.
The embodiment of lactam rings within a wealth of physiologically active natural products and pharmaceutical agents ensures that the development of synthetic methods, which facilitate the preparation of these saturated N-heterocycles, is of critical importance. Herein the development of a versatile method for the synthesis of 4 to 8-membered α-vinyl and α-(2-silylvinyl) lactams involving the iodine(III)-mediated oxidative cyclization of unsaturated O-alkyl hydroxamates, which encompass an allylsilane, is reported. Importantly, the outcome of this transformation can be effectively controlled through variation of the substitution pattern at the silicon center. While allyltrimethylsilanes undergo ring closure with desilylation to form α-vinyl lactams, the corresponding triisopropyl and triphenylsilanes cyclize without loss of the larger silyl group to form E-vinylsilanes with excellent stereoselectivity. From a mechanistic standpoint, it is proposed that this reaction proceeds via concerted alkene addition of a singlet nitrenium ion (or its equivalent) to form a bicyclic N-acyl-N-alkoxyaziridinium ion, which undergoes eliminative ring opening.
Dehydrative Fragmentation of 5-Hydroxyalkyl-1H-tetrazoles: A Mild Route to Alkylidenecarbenes
Duncan J. Wardrop and John P. Komenda
Org. Lett, 2012, 14, 1548-1551
DOI: 10.1021/ol300276p
[Supporting Information]
Duncan J. Wardrop and John P. Komenda
Org. Lett, 2012, 14, 1548-1551
DOI: 10.1021/ol300276p
[Supporting Information]
Abstract
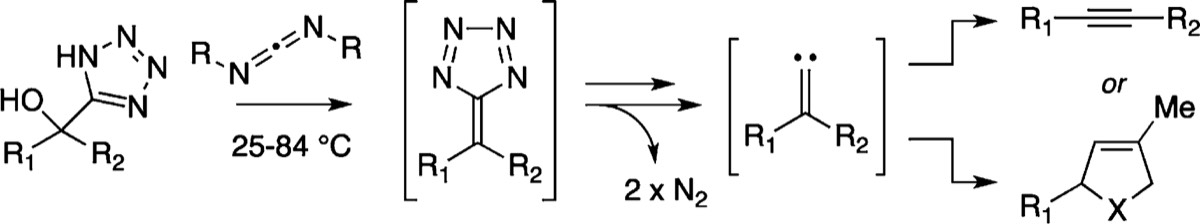
The development of a mild, base-free method for the generation of alkylidenecarbenes is reported. Treatment of 5-hydroxyalkyl-1H-tetrazoles with carbodiimides generates products arising from the 1,2-rearrangement or [1,5]-C–H bond insertion of a putative alkylidenecarbene. Formation of this divalent intermediate is proposed to occur by way of a tetraazafulvene, which undergoes extrusion of 2 mol of dinitrogen. Details of this methodology, its application to the synthesis of combretastatin A-4, and an improved route to 5-hydroxyalkyl-1H-tetrazoles are described.
The development of a mild, base-free method for the generation of alkylidenecarbenes is reported. Treatment of 5-hydroxyalkyl-1H-tetrazoles with carbodiimides generates products arising from the 1,2-rearrangement or [1,5]-C–H bond insertion of a putative alkylidenecarbene. Formation of this divalent intermediate is proposed to occur by way of a tetraazafulvene, which undergoes extrusion of 2 mol of dinitrogen. Details of this methodology, its application to the synthesis of combretastatin A-4, and an improved route to 5-hydroxyalkyl-1H-tetrazoles are described.
Methods for Direct Alkene Diamination, New & Old
Sam de Jong, Daniel G. Nosal and, Duncan J. Wardrop
Tetrahedron 2012, 68, 4067-4105
DOI: 10.1016/j.tet.2012.03.036
Sam de Jong, Daniel G. Nosal and, Duncan J. Wardrop
Tetrahedron 2012, 68, 4067-4105
DOI: 10.1016/j.tet.2012.03.036
Abstract
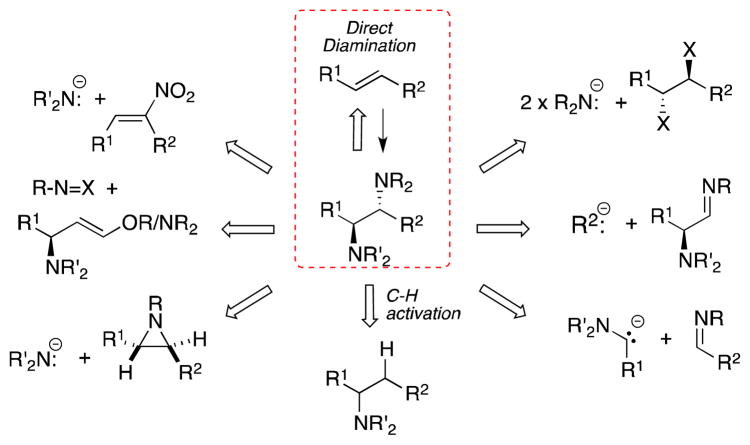
The 1,2-diamine moiety is a ubiquitous structural motif present in a wealth of natural products, including non-proteinogenic amino acids and numerous alkaloids, as well as in pharmaceutical agents, chiral ligands and organic reagents. The biological activity associated with many of these systems and their chemical utility in general has ensured that the development of methods for their preparation is of critical importance. While a wide range of strategies for the preparation of 1,2-diamines have been established, the diamination of alkenes offers a particularly direct and efficient means of accessing these systems. The purpose of this review is to provide an overview of all methods of direct alkene diamination, metal-mediated or otherwise.
The 1,2-diamine moiety is a ubiquitous structural motif present in a wealth of natural products, including non-proteinogenic amino acids and numerous alkaloids, as well as in pharmaceutical agents, chiral ligands and organic reagents. The biological activity associated with many of these systems and their chemical utility in general has ensured that the development of methods for their preparation is of critical importance. While a wide range of strategies for the preparation of 1,2-diamines have been established, the diamination of alkenes offers a particularly direct and efficient means of accessing these systems. The purpose of this review is to provide an overview of all methods of direct alkene diamination, metal-mediated or otherwise.
Discovery, Synthesis, and Biological Evaluation of a Novel Group of Selective Inhibitors of Filoviral Entry
Maria V. Yermolina, Jizhen Wang, Michael Caffrey, Lijun L. Rong, and Duncan J. Wardrop
J. Med. Chem., 2011, 54, 765–781
DOI: 10.1021/jm1008715
Maria V. Yermolina, Jizhen Wang, Michael Caffrey, Lijun L. Rong, and Duncan J. Wardrop
J. Med. Chem., 2011, 54, 765–781
DOI: 10.1021/jm1008715
Abstract
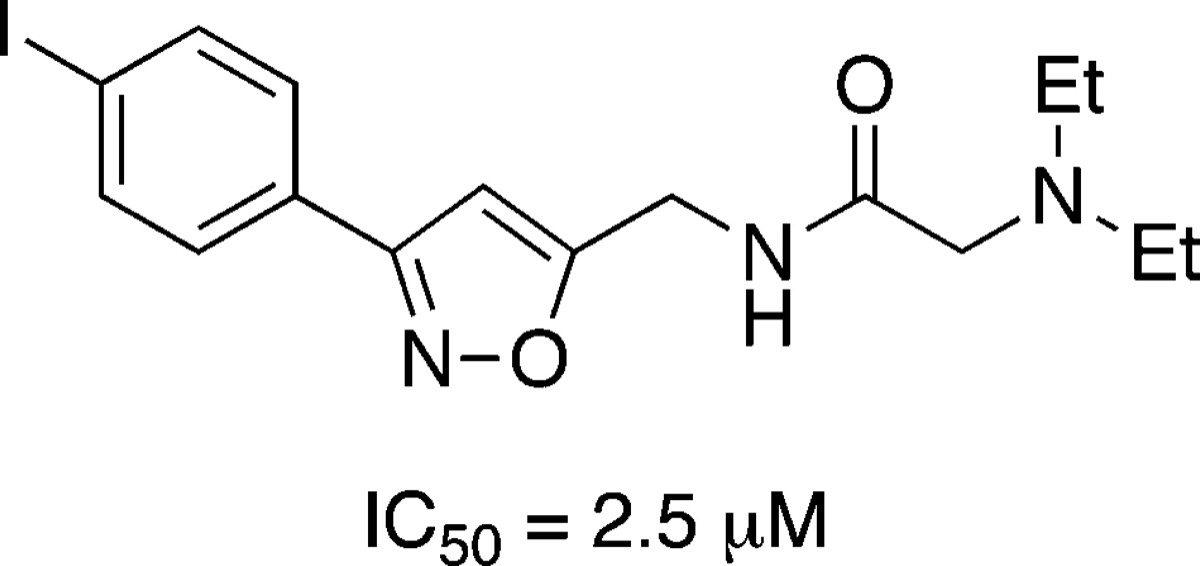
Herein, we report the development of an antifiloviral screening system, based on a pseudotyping strategy, and its application in the discovery of a novel group of small molecules that selectively inhibit the Ebola and Marburg glycoprotein (GP)-mediated infection of human cells. Using Ebola Zaire GP-pseudotyped HIV particles bearing a luciferase reporter gene and 293T cells, a library of 237 small molecules was screened for inhibition of GP-mediated viral entry. From this assay, lead compound 8a was identified as a selective inhibitor of filoviral entry with an IC50 of 30 μM. To analyze functional group requirements for efficacy, a structure−activity relationship analysis of this 3,5-disubstituted isoxazole was then conducted with 56 isoxazole and triazole derivatives prepared using “click” chemistry. This study revealed that while the isoxazole ring can be replaced by a triazole system, the 5-(diethylamino)acetamido substituent found in 8a is required for inhibition of viral-cell entry. Variation of the 3-aryl substituent provided a number of more potent antiviral agents with IC50 values ranging to 2.5 μM. Lead compound 8a and three of its derivatives were also found to block the Marburg glycoprotein (GP)-mediated infection of human cells.
Herein, we report the development of an antifiloviral screening system, based on a pseudotyping strategy, and its application in the discovery of a novel group of small molecules that selectively inhibit the Ebola and Marburg glycoprotein (GP)-mediated infection of human cells. Using Ebola Zaire GP-pseudotyped HIV particles bearing a luciferase reporter gene and 293T cells, a library of 237 small molecules was screened for inhibition of GP-mediated viral entry. From this assay, lead compound 8a was identified as a selective inhibitor of filoviral entry with an IC50 of 30 μM. To analyze functional group requirements for efficacy, a structure−activity relationship analysis of this 3,5-disubstituted isoxazole was then conducted with 56 isoxazole and triazole derivatives prepared using “click” chemistry. This study revealed that while the isoxazole ring can be replaced by a triazole system, the 5-(diethylamino)acetamido substituent found in 8a is required for inhibition of viral-cell entry. Variation of the 3-aryl substituent provided a number of more potent antiviral agents with IC50 values ranging to 2.5 μM. Lead compound 8a and three of its derivatives were also found to block the Marburg glycoprotein (GP)-mediated infection of human cells.
Nitrenium Ion-Mediated Alkene Bis-Cyclofunctionalization: Total Synthesis of (−)-Swainsonine
Duncan J. Wardrop and Edward G. Bowen
Org. Lett., 2011, 13, 2376-2379
DOI: 10.1021/ol2006117
[Supporting Information]
Duncan J. Wardrop and Edward G. Bowen
Org. Lett., 2011, 13, 2376-2379
DOI: 10.1021/ol2006117
[Supporting Information]
Abstract
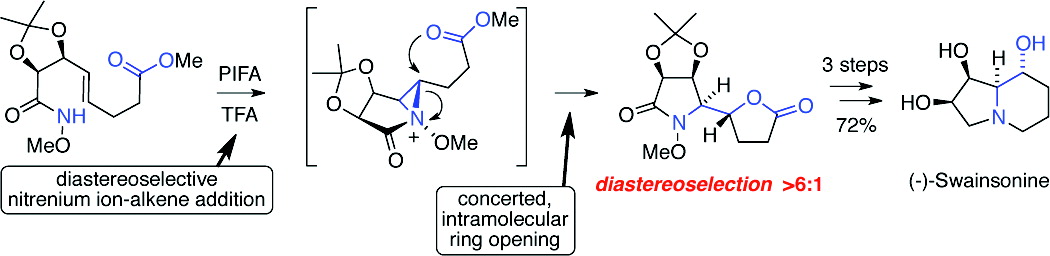
The total synthesis of (−)-swainsonine from 2,3-O-isopropylidene-d-erythrose in 12 steps and an overall yield of 28% is reported. The pivotal transformation in our route to this indolizidine alkaloid is the formation of the pyrrolidine ring and C-8a/8 stereodiad through the diastereoselective, bis-cyclofunctionalization of an γ,δ-unsaturated O-alkyl hydroxamate. This transformation is believed to proceed via the intramolecular capture of an N-acyl-N-alkoxyaziridinium ion generated by the diastereoselective addition of a singlet acylnitrenium ion to the pendant alkene.
The total synthesis of (−)-swainsonine from 2,3-O-isopropylidene-d-erythrose in 12 steps and an overall yield of 28% is reported. The pivotal transformation in our route to this indolizidine alkaloid is the formation of the pyrrolidine ring and C-8a/8 stereodiad through the diastereoselective, bis-cyclofunctionalization of an γ,δ-unsaturated O-alkyl hydroxamate. This transformation is believed to proceed via the intramolecular capture of an N-acyl-N-alkoxyaziridinium ion generated by the diastereoselective addition of a singlet acylnitrenium ion to the pendant alkene.
Synthesis and Biological Activity of Naturally Occurring α-Glucosidase Inhibitors
Duncan J. Wardrop and Samanthi L. Waidyarachchia
Nat. Prod. Rep., 2010, 27, 1431-1468
DOI: 10.1039/B914958A
Duncan J. Wardrop and Samanthi L. Waidyarachchia
Nat. Prod. Rep., 2010, 27, 1431-1468
DOI: 10.1039/B914958A
Abstract
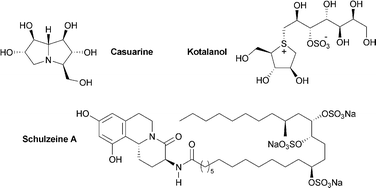
In addition to their clinical importance in the treatment of type II diabetes, a-glucosidase inhibitors have attracted considerable attention from the synthetic community because of their profound effect on an array of cellular processes, including N-linked glycoprotein processing and maturation, oligosaccharide metabolism, and cell–cell and cell–virus recognition. Over the past decade, a number of structurally novel naturally occurring alpha-glucosidase inhibitors which do not conform to the classical iminosugar mold have been identified, including zwitterionic thiosugars and marine organosulfates. While these natural products are important leads in the development of new classes of glucosidase inhibitors, they have also attracted considerable attention as synthetic targets in of themselves. This article reviews the recent literature concerning the synthesis of these emerging natural product families, as well as the preparation of those polyhydroxylated alkaloids more traditionally associated with anti-a-glucosidase activity. 176 references are cited.
In addition to their clinical importance in the treatment of type II diabetes, a-glucosidase inhibitors have attracted considerable attention from the synthetic community because of their profound effect on an array of cellular processes, including N-linked glycoprotein processing and maturation, oligosaccharide metabolism, and cell–cell and cell–virus recognition. Over the past decade, a number of structurally novel naturally occurring alpha-glucosidase inhibitors which do not conform to the classical iminosugar mold have been identified, including zwitterionic thiosugars and marine organosulfates. While these natural products are important leads in the development of new classes of glucosidase inhibitors, they have also attracted considerable attention as synthetic targets in of themselves. This article reviews the recent literature concerning the synthesis of these emerging natural product families, as well as the preparation of those polyhydroxylated alkaloids more traditionally associated with anti-a-glucosidase activity. 176 references are cited.
Diastereoselective Nitrenium Ion-mediated Cyclofunctionalization: Total Synthesis of (+)-Castanospermine
Duncan J. Wardrop and Edward G. Bowen
Org. Lett., 2010, 12, 5330-5333
DOI: 10.1021/ol102371x
[Supporting Information]
Duncan J. Wardrop and Edward G. Bowen
Org. Lett., 2010, 12, 5330-5333
DOI: 10.1021/ol102371x
[Supporting Information]
Abstract
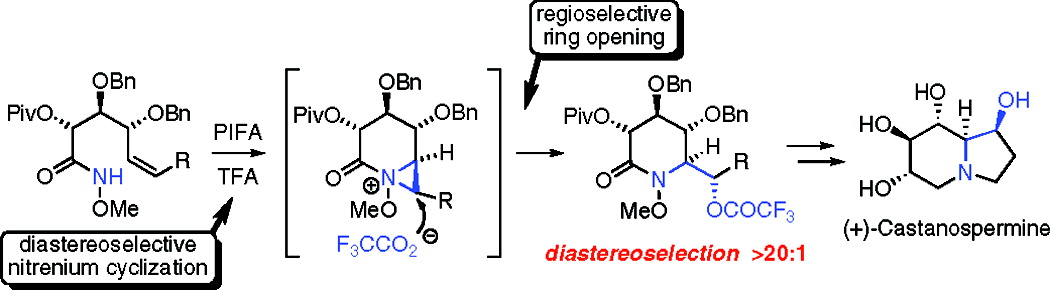
The asymmetric total synthesis of the α-glucosidase inhibitor (+)-castanospermine is reported. The central theme in our approach to this polyhydroxylated alkaloid is the simultaneous generation of the piperidine ring and the C-1/8a erythro stereodiad through the diastereoselective, oxamidation of an unsaturated O-alkyl hydroxamate. This process is believed to proceed sequentially via singlet acylnitrenium and aziridinium ion intermediates.
The asymmetric total synthesis of the α-glucosidase inhibitor (+)-castanospermine is reported. The central theme in our approach to this polyhydroxylated alkaloid is the simultaneous generation of the piperidine ring and the C-1/8a erythro stereodiad through the diastereoselective, oxamidation of an unsaturated O-alkyl hydroxamate. This process is believed to proceed sequentially via singlet acylnitrenium and aziridinium ion intermediates.
Intramolecular Oxamidation of Unsaturated O-Alkyl Hydroxamates: A Remarkably Versatile Entry to Hydroxy Lactams
Duncan J. Wardrop, Edward G. Bowen, Raymond E. Forslund, Adam D. Sussman and Samanthi L. Weerasekera
J. Am. Chem. Soc., 2010, 132, 1188–1189
DOI: 10.1021/ja9069997
[Supporting Information]
Duncan J. Wardrop, Edward G. Bowen, Raymond E. Forslund, Adam D. Sussman and Samanthi L. Weerasekera
J. Am. Chem. Soc., 2010, 132, 1188–1189
DOI: 10.1021/ja9069997
[Supporting Information]
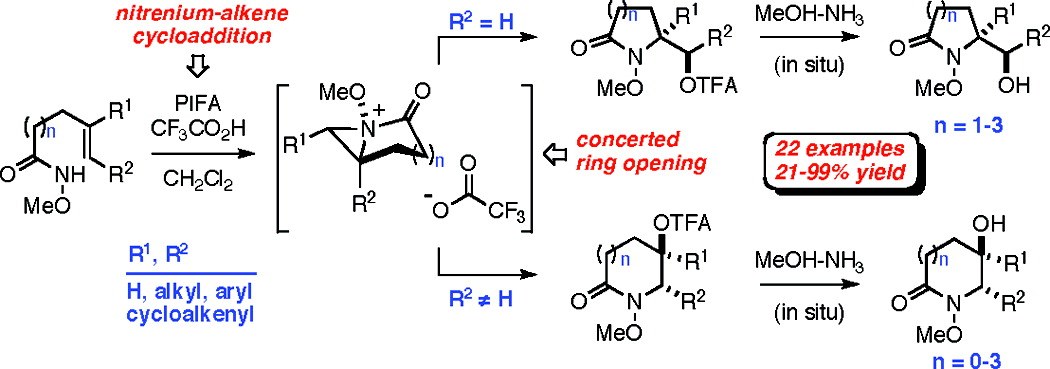
Abstract
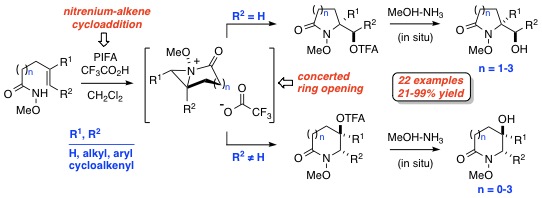
The development of a versatile method for the preparation of five- to eight-membered hydroxy lactams that involves the iodine(III)-mediated oxamidation of unsaturated O-alkyl hydroxamates is described. This transformation, which is believed to proceed through the intermediacy of singlet nitrenium and bicyclic N-acyl-N-alkoxyaziridinium ions, is both stereospecific and highly regioselective in most of the 22 cases examined.
The development of a versatile method for the preparation of five- to eight-membered hydroxy lactams that involves the iodine(III)-mediated oxamidation of unsaturated O-alkyl hydroxamates is described. This transformation, which is believed to proceed through the intermediacy of singlet nitrenium and bicyclic N-acyl-N-alkoxyaziridinium ions, is both stereospecific and highly regioselective in most of the 22 cases examined.
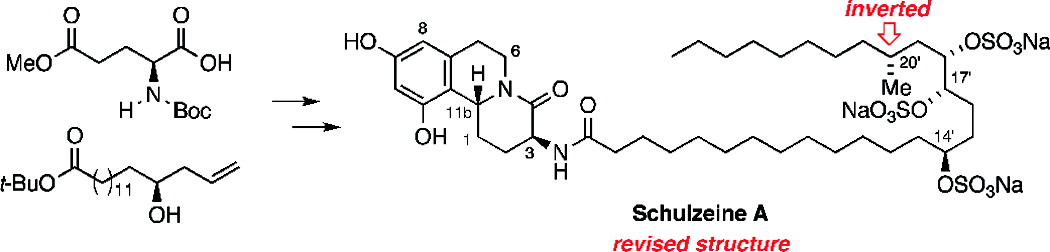
Total Synthesis of the α-Glucosidase Inhibitors Schulzeine A, B, and C and a Structural Revision of Schulzeine A
Duncan J. Wardrop and Edward G. Bowen
J. Am. Chem. Soc., 2009, 131, 6062–6063
DOI: 10.1021/ja9005755
[Supporting Information]
Duncan J. Wardrop and Edward G. Bowen
J. Am. Chem. Soc., 2009, 131, 6062–6063
DOI: 10.1021/ja9005755
[Supporting Information]
The enantioselective total synthesis of the potent alpha-glucosidase inhibitors schulzeine A, B, and C and a revision of the proposed C20' configuration of schulzeine A are reported. The central feature of our convergent route to this family of novel marine natural products is the preparation of the common benzo[a]quinolizidine subunit through a substrate-controlled, diastereoselective Pictet-Spengler cyclocondensation.
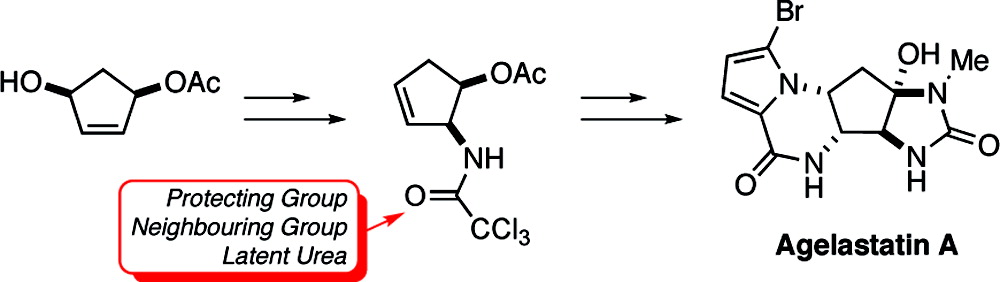
Total Synthesis of (±)-Agelastatin A, A Potent Inhibitor of Osteopontin-Mediated Neoplastic Transformations
David P. Dickson and Duncan J. Wardrop
Org. Lett, 2009, 11, 1341-1344
DOI: 10.1021/ol900133v
[Supporting Information]
David P. Dickson and Duncan J. Wardrop
Org. Lett, 2009, 11, 1341-1344
DOI: 10.1021/ol900133v
[Supporting Information]
Abstract
A stereoselective synthesis of agelastatin A, a potent cytotoxin and inhibitor of osteopontin (OPN)-mediated neoplastic transformations, has been accomplished in 14 steps (12 operations) with an approximate overall yield of 8%. Notable features of this route include the direct manner in which the pyrroloketopiperazine A-ring of the target is generated and the efficient employment of a trichloroacetamide, introduced through Overman rearrangement, as a protecting group, pendant nucleophile, and latent urea.
A stereoselective synthesis of agelastatin A, a potent cytotoxin and inhibitor of osteopontin (OPN)-mediated neoplastic transformations, has been accomplished in 14 steps (12 operations) with an approximate overall yield of 8%. Notable features of this route include the direct manner in which the pyrroloketopiperazine A-ring of the target is generated and the efficient employment of a trichloroacetamide, introduced through Overman rearrangement, as a protecting group, pendant nucleophile, and latent urea.
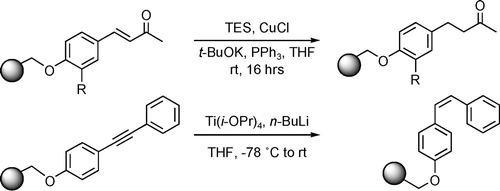
Reduction of Solid-Supported Olefins and Alkynes
David P. Dickson, Christine Toh, Menaka Lunda, Maria V. Yermolina, Duncan J. Wardrop and Chad L. Landrie
J. Org. Chem., 2009, 74, 9535–9538
DOI: 10.1021/jo901764u
[Supporting Information]
David P. Dickson, Christine Toh, Menaka Lunda, Maria V. Yermolina, Duncan J. Wardrop and Chad L. Landrie
J. Org. Chem., 2009, 74, 9535–9538
DOI: 10.1021/jo901764u
[Supporting Information]
Abstract
The reduction of carbon−carbon multiple bonds in alkynes and olefins supported on a polystyrene resin has been investigated. Homogeneous catalysis by titanocene reagents is effective for the stereoselective preparation of cis-olefins from diarylacetylenes, while the use of copper(I) hydride reagents is effective for the reduction of α,β-unsaturated ketones.
The reduction of carbon−carbon multiple bonds in alkynes and olefins supported on a polystyrene resin has been investigated. Homogeneous catalysis by titanocene reagents is effective for the stereoselective preparation of cis-olefins from diarylacetylenes, while the use of copper(I) hydride reagents is effective for the reduction of α,β-unsaturated ketones.
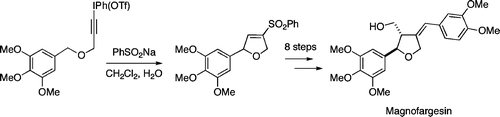
Total Synthesis of (±)-Magnofargesin
Duncan J. Wardrop and Joseph Fritz
Org. Lett., 2006, 8, 3659-3662
DOI: 10.1021/ol0609053
[Supporting Information]
Duncan J. Wardrop and Joseph Fritz
Org. Lett., 2006, 8, 3659-3662
DOI: 10.1021/ol0609053
[Supporting Information]
Abstract
A convenient method for the preparation of 2,5-dihydrofurans by using the chemistry of alkynyl(phenyl)iodonium salts is reported. Treatment of 3-alkoxy-1-alkynyl(phenyl)iodonium triflates with sodium benzenesulfinate generates an alkylidenecarbene, which undergoes [1,5]-C-H insertion to form 2-substituted 4-benzenesulfonyl-2,5-dihydrofurans in reasonable yield. These cyclic vinyl sulfones are functionalized in such a way as to make them useful starting materials for the preparation of lignans. The application of this methodology to the first total synthesis of (±)-magnofargesin is disclosed.
A convenient method for the preparation of 2,5-dihydrofurans by using the chemistry of alkynyl(phenyl)iodonium salts is reported. Treatment of 3-alkoxy-1-alkynyl(phenyl)iodonium triflates with sodium benzenesulfinate generates an alkylidenecarbene, which undergoes [1,5]-C-H insertion to form 2-substituted 4-benzenesulfonyl-2,5-dihydrofurans in reasonable yield. These cyclic vinyl sulfones are functionalized in such a way as to make them useful starting materials for the preparation of lignans. The application of this methodology to the first total synthesis of (±)-magnofargesin is disclosed.