 See
table 9.1 OH 7
See
table 9.1 OH 7Chapter 9 AP Chemistry Notes
See p. 277
2 Models
1)
A. O. [Atomic Orbital Hybridization or Valence Bond]2)
M. O. [Molecular Orbital]p. 278
We know geometry from absorption spectra, electron diffractionValence Shell Pair Repulsion
See p. 278 & Fig. 9.1
See your periodic table with single bonds we get on table
See Fig. 9.3 (OH 2,3,4,5)
Unshared Pair
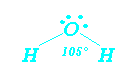
Fig. 9.4 (OH 6)Another classic is H2O bent
 See
table 9.1 OH 7
See
table 9.1 OH 7
See p. 284 #s 1 and 2, p. 286
Expanded Octet Models
XeF6
p. 309? OH 8See p. 287 Underline and bottom summary p. 288 very nice
Polarity
d+
H:Cl d-m
[Dipole moment] = the magnitude of the charge displacement in a polar bond3.34x10
-30 C*m = 1 DeBye (D)
HCl
m = 1.030 = 16% ionic characterRead hand out on Polar Bonds
Demo some
Atomic Orbitals
Valence Bond Theory
Hybrid Orbitals
1) SP [Group 2]
p. 294 Fig. 9.9Classic Example BeF2
2) SP
2 [Group 13] p.294 Classic Example BF33) SP3 [Group 14]
p. 295Classic Example CH4
Group 15 and 16 SP3 also at times
4) SP3 D expanded octet
p. 295Classic example PCl5
5) SP3 D2
Classic example SF6, XeF4
Here are some basic molecular configurations
Here are some animated molecular configurations
See p. 296 Table 9.3
Links:
Atomic Orbitals: http://www.albany.net/~cprimus/orb/index.html
Atomic Orbitals...again: http://chipo.chem.uic.edu/web1/ocol/SB/1-2.htm
Back to the Class Notes Homepage
|
|
|