

Chapter 8
What types of elements have covalent bonds?
See p. 250
The Interaction of Two Hydrogen Atoms
Hydrogen atoms


![]()
sufficiently far apart
to have no interaction

![]()
optimum distance to achieve lowest
overall energy of system

H2 molecule
H:H
See p. 253 (bold)
Unshared Pair
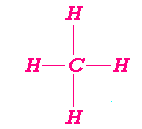
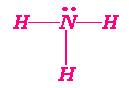
Octet Rules
Know p. 254Highlight Central Atom
See p. 258
Resonance Forms NO3-
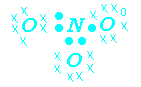
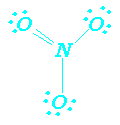
![]() coordinate covalent bond
coordinate covalent bond
Click here for a great site on Resonance
See p. 266à 269
Circle p. 258 1,2,3
Do Ex 21 ab, 22 p.274
Know isomers C2H2Cl2
EXCEPTIONS!!!
p. 259
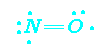 odd electrons
odd electrons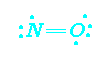
NO and NO2 are classic examples
See p. 260
The B family
The Be family
See OH 6
Expanded Octets:
See Table p. 261PCl5 , SF6 , XeF4 are classics
The only true nonpolar bonds are?_________
Polar Means:
See OH 7,8
Know how to do Exp. 2 Ex. 8.5 without Table 8.2 but just chart!
If bonds in the product molecules are stronger than the reactants, the products are more stable and have lower energy.
"Weak" Bonds à "Strong" Bonds
DH<0"Strong" Bonds à "Weak" Bonds
DH>0
SINGLE BOND ENERGIES (kJ/mol) at 25oC

See p.270 (in red)
Do Ex p.270
p.271 summary
Back to the Class Notes Homepage
 |
|