CLOSED
Chapter 7
THERMODYNAMICS
Fundamental Definitions
System:
That part of the universe within some definable boundariesSurroundings:
Everything in the universe outside of the systemTypes of Systems
Open System:
A system that can exchange both matter and energy with the surroundingsClosed System:
A system that can exchange energy, but not matter with the surroundingsIsolated System:
A system that cannot exchange either matter or energy with the surroundingsPractice Problem on Classifying Different
Types of Systems
Classify each of these systems as open, closed, or isolated
A thin-walled glass sphere
CLOSED
A beaker containing a solution
OPEN
 A well-insulated, sealed box
A well-insulated, sealed box
ISOLATED
Note that an isolated system would have no way of knowing that the rest of the universe even existed!
Some very fundamental concepts regarding the essential nature of thermodynamics.
STATE FUNCTIONS
The thermodynamic description of a system is accomplished by specifying the values of certain quantities called
state functions.State functions have two important properties.
Common State Functions and Their Accepted Symbols
Volume V
Temperature T (always in K)
Pressure P
Energy E
Enthalpy H
Entropy S
Free Energy G
Example: State Functions and Some Changes
For one mole of an ideal gas
Initial State
P1 = 1.0 atm V1 = 22.4 L T1 = 273 K
Final State
P2 = 10.0 atm V2 = 4.48 L T2 = 546 K
Changes
D
P = P2 - P1 = 10.0 – 1.0 = 9.0 atmD
V = V2 - V1 = 4.48 – 22.4 = -17.9LD
T = T2 - T1 = 546 – 273 = 273 KImportant Convention
A change in a thermodynamic quantity is always defined as the final state minus the initial state. Adherence to correct sign conventions is critical when working thermodynamics problems.
Example:
D
T = TF – TI
HEAT CAPACITY
(Know page 211)
Heat Capacity
: The amount of energy required to change the temperature of a material by one Celcius (or Kelvin) degree. (Units J/o C)Note: A related quantity which really measures the same thing, is something called the
specific heat of a substance, which is the amount of energy required to change the temperature of one gram of a material by one Celcius (or Kelvin) degree. (Units J/g o C)Practice Problem
The specific heat of solid iron is .4570 J/g o C. If 35.0 g of pure iron, initially at a temperature of 150.0 o C, is added to 125.0 mL of pure water, initially at a temperature of 25.0 o C, what will be the final temperature reached by the system?
Assume a specific heat for water of 4.184 J/g o C and a density of 1.00g/mL, and that no heat is lost to the surroundings.
Let the final temperature reached = TF
(When thermal equilibrium is reached)
Heat lost by Fe = Heat gained by H2O
Heat à
DE = M x DT x S.H.
TiFe = 150.0 o C TiH2O = 25.0 o C
MFe = 35.0g MH2O = 125.0g
S.H.Fe = .4570 J/g o C S.H.H2O = 4.184 J/g o C
-qFe = qH2O
-[ MFe x (TF – Ti
) x S.H.Fe ] = MH2O x (TF – Ti ) x S.H.H2OOk pal work it out now!
The answer is 28.7o C
D
E = M x DT x S.H. = qDT = (TF –Ti
)S.H.
H2O = 4.184J/g 0 C = 1.00 cal/g 0 CSee table 7.1à
Water has a high S.H. (p. 213)Exothermicà q<0 Endothermicà q>0
See p. 215
qReaction = -qcal
See OH#9
qReaction = -[Ccal ] x
DTCcal is really Heat Capacity J/0 C (p. 216)
Enthalpy (H) = Heat Content
qReaction =
DH = HP – HRtherefore, exothermicà
DH<0; endothermicà DH>0C2H5OH + 7/2O2 à 2CO2 + 3H2O
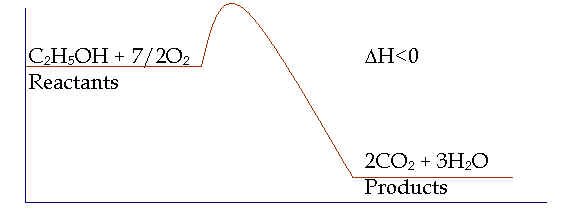
Reaction Path
See p. 218à Note law of conservation of energy
C2H5OH(l) + 7/2O2(g) à 2CO2(g) + 3H2O(g) + 1238.7 kJ/mol
Laws of Thermodynamics
2CO2(g) + 3H2O(g) + 1238.7 kJ/molà C2H5OH(l) + 7/2O2(g)
D
H = 1238.7 kJ/molThe Second Law of Thermodynamics LINK!
Back to the Class Notes Homepage

Maintained by: Chris Weilemann
Lee Marek