AP
Chemistry - Chapter 5 Notes
The chemical properties of atoms are
determined by the way in which the e- are arranged about the
nucleus. If we know the electronic structure of an atom, we can predict much of
the chemical behavior of the element(s). That is the point of this
chapter.
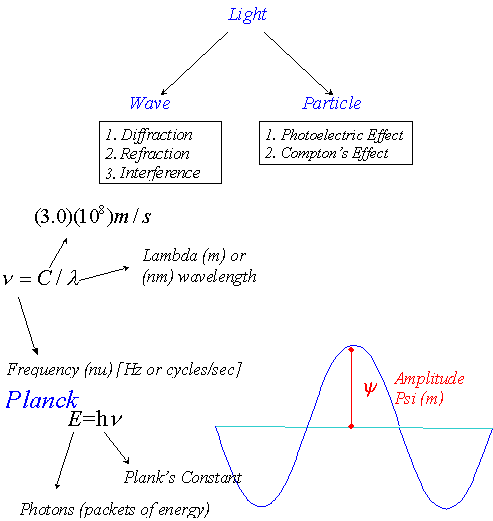
Blackbody Radiation
Prior to 1900, light was assumed to simply be an electromagnetic wave. As such, the energy carried by a light wave was proportional to the sum of the squares of the amplitudes of the electric and magnetic waves.
E a (E2 mass + H2 max) a Light intensity
Notice that the frequency of a light wave has absolutely nothing to do with the energy carried by the light.
The chemical properties of atoms are determined by the way in which the e- are arranged about the nucleus.
Results of Blackbody Radiation Experiments

Planck’s Explanation
![]()
where n is a positive integer, and h is Planck’s constant.
![]()
Know: n =c/l & E=hn

Balmer’s Formula – 1885
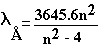 |
where n = 3,4,… |
or
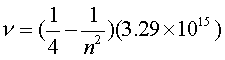
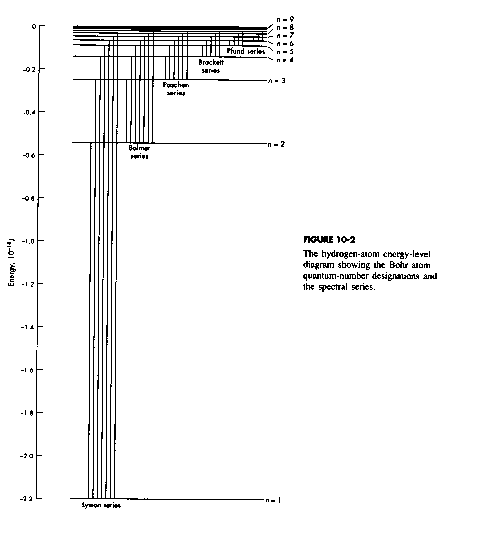
Spectrum of the H-Atom
There are several groups of lines all following the general qualitative pattern.
Ultraviolet – Lyman Series
Visible – Balmer Series
Infrared – Paschen Series
|
Lyman Series Energies
984.1 kJ/mol |
Balmer Series 182.2 kJ/mol |
It took something / someone to go from here, someone to make a paradigm shift, to show that matter can act as a wave and as a particle.
Bohr was the "intermediate" between the "old" physics (Newton physics) and the "new" quantum physics."
Predestination vs. Determinism
The main contribution of Bohr, as we shall see, was to devise a model for the atom and demonstrate, by the aid of a model, that he could account for spectral lines in terms of energy levels within the atom.
However, there was one slight limitation…
it only worked for 1 e- (electron) systems!
BOHR POSTULATED: Radius of an orbit is fixed by the requirement that the force of attraction between a nucleus or charge Ze, an electron of charge e- is given by COULOMB’S LAW
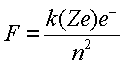 |
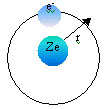 |
Also, the centripetal force of the electron moving in an orbit may be written as…
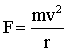 |
m = mass of e- |
We have two forces again:
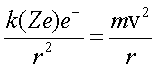
Now Bohr imposed a Quantum Condition because it worked (hard to accept that)!
| Angular Momentum | mvr = constant |
|
|
|
n = 1, 2, 3, … positive whole numbers
h = Planck’s Constant
Bohr found:
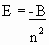
See p. 138
De Broglie. His idea brought matter and waves together. He said, matter, like light or any E.M.R. can act as both a wave and a particle.
Louis de Broglie’s Derivations
Plank Einstein
E= hn E=mc2
SO setting them = hn =mc2
replacing n =c/l
we get hc/l =mc2
or
l=h/mc
Matter does not move at the sound of light…
soooo l = h/mc becomes l = h/mv
l = h/mv
where V is speedMatter waves!
2p r = nh/mvl
mvr = nh/2p
What does this mean?!
See p.142
One needs a fixed number of waves or else one will get interference of waves. The waves have to fit on a circle so you need a whole number of waves.
nλ = 2πr
"Proved" by Davisson and Germer in 1927 at Bell Labs when they found diffraction patterns for e- just like light:
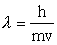 (note:
the larger the object, the smaller the l )
(note:
the larger the object, the smaller the l )
Heisenberg Uncertainty Principle
The inability to predict both the exact location of atomic particles and their momenta at the same time:
(D P)(D x) » h
D P = momentum = mv D x = distance
So, we end up speaking about where the e- is and its momentum in terms of probability
A new model had to be developed:
Quantum Mechanics
We need to use Probability!
See p. 132, 133 Use (d - Salt)
D E = hn =h(c/l ) l =h/(mv)
Know these 3 equations!
Wave model developed by Schröedinger flooded planet model.
Wave characteristics to electrons (p. 144)
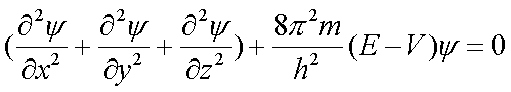
x, y, z
ą space coordinates m ą mass of an electron (e-)E ą total energy of electron-proton system
V ą potential energy
y ą (Greek letter, psi) amplitude of wave function
Solutions yield expressions for y and certain quantum numbers pop out. These values determine the allowed energy states of the electrons and the shapes of the space the electrons occupy.
y2 is the probability of finding the electron in an element of unit volume about the nucleus.
y 2 µ the electron cloud density
Electron clouds are probability regions in space where the electron (e-) is likely to be found. We call these orbitals. Each orbital has associated with it a set of quantum numbers (like an address):
(n, ℓ, mℓ)
These come out of the Schröedinger equation (see p. 145)
Wave model developed by Schröedinger flooded planet model.
Wave characteristics to electrons (p. 144)
![]() n
n
The value of n determines to a large extent the ENERGY of an e- and is related to the average DISTANCE of an e- from the nucleus. (Also periods in table)
n=1 is the lowest
n comes in positive whole numbers
In a given energy level there are 2n2 e-
As n![]() , E
, E![]() , and as
n
, and as
n![]() , Volume
, Volume![]()
n also indicates the number of nodal surfaces associated with each orbital.
![]()
| Location where probability of finding an e- is zero. |
Is directly related to the SUBLEVEL and hence the SHAPE of the orbitals
ℓ
l is determined by n. It may take on values of 0 to n – 1.
n = 1 ℓ = 0
n = 2 ℓ = 0 or 1
n = 3 ℓ = 0, 1, or 2
n = 4 ℓ = 0, 1, 2, or 3
Value of ℓ Sublevel Type Maximum # of e- 0 s 2 1 p 16 2 d 10 3 f 14
![]() mℓ
Gives us the directional characteristics of the sublevels
(Š )
mℓ
Gives us the directional characteristics of the sublevels
(Š )
mℓ takes on the values of + ℓ, …, 0, …, – ℓ.
|
l |
ml |
Sublevel |
|
0 |
0 |
s |
|
1 |
+1, 0, -1 |
pxpypz |
|
2 |
+2, +1, 0,-1, -2 |
d’s |
In a given sublevel there are 2 l +1 orbitals. ml tells us what orbital we are in.
Cool site for f orbitals see http://www.albany.net/~cprimus/orb
![]() ms
ms
ms takes on values of +1/2 or –1/2
PAULI EXCLUSION PRINCIPLE
(Know it!!!) See p. 149 all red
See OH 28
See p. 152 Fig. 5.11


Electron Configuration
AUFBAU
(Building Up)![]() Electron Box Diagram
Electron Box Diagram
See. P. 153
See OH 29, 30, 31, 32
HUND’S RULE See p. 155, 156
Monatomic Ions - Representative Elements
- Transition elements
- See p. 159
![]()
Back to the Class Notes Homepage
|
|
|