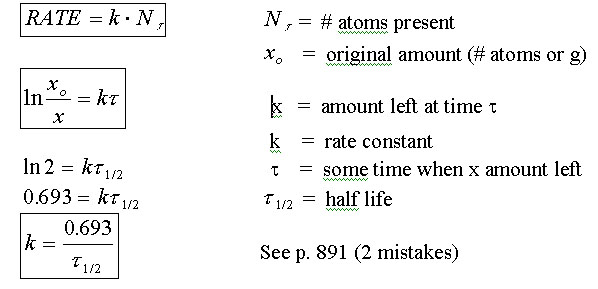
|
|
||
|
The Players a Proton See Figure 25.1
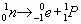
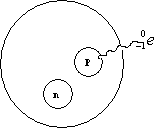 |
Know a , b , and g decay. These occur naturally in elements above 83. 
If
If |
|
|
|
||
|
The Players a Proton See Figure 25.1
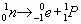
 |
Know a , b , and g decay. These occur naturally in elements above 83. 
If
If |
|
Know p. 882 a , b , g
Know INDUCED RADIOACTIVITY
Elements
above 94 are man-made by neutron bombardment or ![]() or
others.
or
others.
NOTE: Show p. A-17 (how to do ln)
![]() ;
;
![]()
Also see
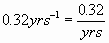
NUCLEAR
DECAY
a
PROBLEM:
The half life of Mareksteinium is 39.2 years. If you have 75.2 g of it, how much will be left in 100. years?
GIVEN: m
= 75.2 g t
= 100. years
![]() = 39.2 years
= 39.2 years
FIND: amount left
Use 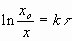 . So we need to calculate:
. So we need to calculate:
m = ![]() and
x
=
amount left
and
x
=
amount left
We also need k:
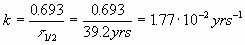
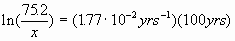
See p. 900: Reaction 25.22, 25.23, 25.24
FISSION: Be able to recognize it ® the splitting of a heavy isotope by bombardment with a neutron into 2 middle weight isotopes and more neutrons + energy
(see other OH’s)
see OH - 6
FUSION: The joining of light nuclei to form heavy ones with the release of ENERGY
You have to have very high temperatures (100 m° ). You have the PLASMA STATE where "e" are ripped off. Ninety-nine percent of matter in the universe is this.
In both FUSION and FISSION small amounts of mass are LOST (products < reactants) - it goes into energy via:
If I had a donkey and a cow...I could make a cowkey!
Back to the Class Notes Homepage
 |
|