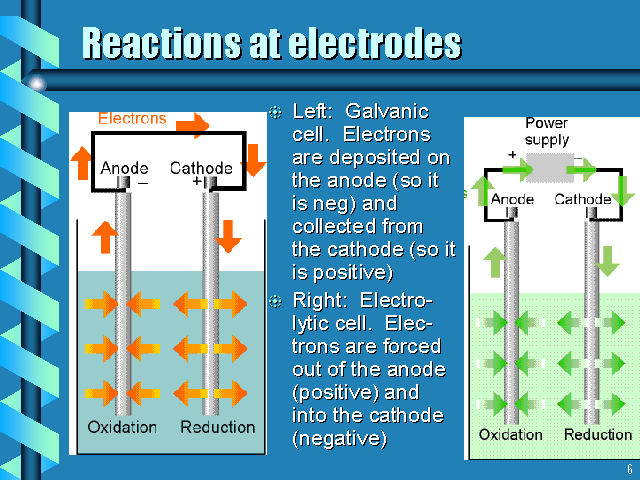
This image is from http://www.westga.edu/~chem/courses/chem410/3510_06/sld006.htm
Chapter 21
What are the 2 kinds of cells?
p. 731

This image is from http://www.westga.edu/~chem/courses/chem410/3510_06/sld006.htm
What is oxidation, reduction anode cathode?
(See OH # 2 & 2-1)
What is a volt?
J/Coulomb
What is a coulomb (C)?
Charge
1 Mole e- = 96, 500 C= F
(A Faraday) ¿
What is an Ampere?
C/s
Q= I*t
Charge = Current *
Time
I. Voltaic Cell: A spontaneous reaction used to produce electric energy
Zn/Zn2+ // Cu2+/Cu
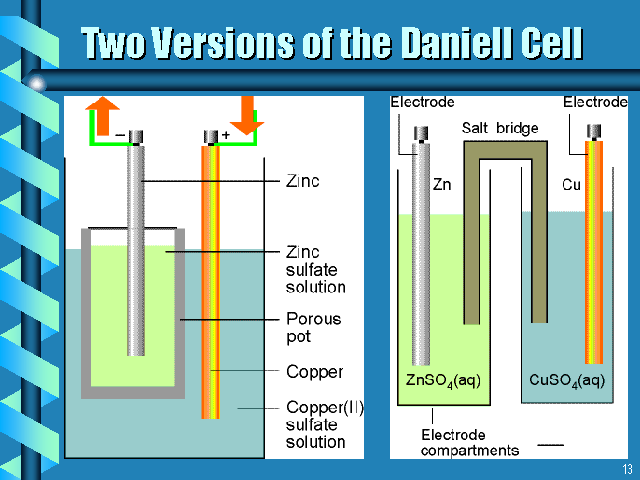
Also from http://www.westga.edu/~chem/courses/chem410/3510_06/sld006.htm
See pg.734 Know by heart!
See OH #3
Salt bridge allows current to flow but prevents contact between Zn and Cu2+, which would short-circuit the cell.
See Fig. 21.3 p. 735 OH 5.
Read 5 items p. 737
What is S.H.E.? Why have it?
Can’t measure single ½ pot.
Standard Hydrogen Electron at 1Atm, 1M[H+], 25° C
2H+ + 2e- -> H2(g) E0red = ZERO, "0", Volts
This
can also be found at http://www.westga.edu/~chem/courses/chem410/3510_06/sld007.htm
See table 21.1 (OH 6)
|
Half-Reaction |
Eored (V) |
|
Li+ + e- à Li |
-3.040 |
|
K+ + e- ® K |
-2.936 |
|
Ba2+ + 2 e- ® Ba |
-2.906 |
|
Ca2+ + 2 e- ® Ca |
-2.869 |
|
Na+ + e- ® Na |
-2.714 |
|
Mg2+ + 2 e- ® Mg |
-2.357 |
|
2H2O(l) ® 4e– + 4H+(aq) + O2(g) |
-1.23V |
|
Al3+ + 3 e- ® Al |
-1.68 |
|
Mn2+ + 2 e- ® Mn |
-1.182 |
|
Fe(OH)2(s) + 2e- ® Fe(s) + 2OH-(aq) |
-0.891 |
|
2H2O + 2e- ® H2(g) + 2OH-(aq) |
-0.828 |
|
Zn2+ + 2 e- ® Zn |
-0.762 |
|
Cr3+ + 3 e- ® Cr |
-0.744 |
|
Fe(OH)3(s) + e- ® Fe(OH)2(s) + OH-(aq) |
-0.547 |
|
S + 2 e- ® S2- |
-0.445 |
|
Fe2+ + 2 e- ® Fe |
-0.409 |
|
Cr3+ + e- ® Cr2+ |
-0.408 |
|
Cd2+ + 2e- à Cd |
-0.402 |
|
PbSO4 (s) + 2e- à Pb(s) + SO42- |
-0.356 |
|
Tl+ + e- à Tl |
-0.336 |
|
Co2+ + 2 e- ® Co |
-0.282 |
|
Ni2+ + 2 e- ® Ni |
-0.236 |
|
AgI(s) + e- à Ag(s) + I-(aq) |
-0.152 |
|
Sn2+ + 2 e- ® Sn |
-0.141 |
|
NO3-(aq) + 2H2O + 3e- ® NO(g) + 4OH-(aq) |
-0.140 |
|
Pb2+ + 2 e- ® Pb |
-0.127 |
|
2 H+ + 2 e- ® H2 |
0.000 |
|
NO3-(aq) + H2O + 2e- ® NO2(g) + 2OH-(aq) |
0.004 |
|
AgBr(s) + e- à Ag(s) + Br-(aq) |
0.073 |
|
S + 2H+ +2e- à H2S |
0.144 |
|
Sn4+ + 2 e- ® Sn2+ |
0.154 |
|
SO42- + 4H+ + 2e- à SO2(g) + 2H2O |
0.155 |
|
Cu2+ + e- ® Cu+ |
0.161 |
|
ClO4-(aq) + H2O + 2e- ® ClO3-(aq) + OH- |
0.398 |
|
Cu2+ + 2 e- ® Cu |
0.339 |
|
O2 + 2 H2O + 4 e- ® 4 OH- |
0.401 |
|
Cu+ + e- ® Cu |
0.518 |
|
I2 + 2 e- ® 2 I- |
0.534 |
|
ClO3-(aq) + 3H2O + 6e- ® Cl-(aq) + 6OH- |
0.614 |
|
O2 + 2 H+ + 2 e- ® H2O2 |
0.680 |
|
Fe3+ + e- ® Fe2+ |
0.769 |
|
Hg22+ + 2 e- ® 2Hg(l) |
0.796 |
|
Ag+ + e- ® Ag |
0.799 |
|
ClO-(aq) + H2O + 2e- ® Cl-(aq) + 2OH- |
0.890 |
|
2Hg2+ + 2 e- ® Hg22+ |
0.908 |
|
NO3- + 4H+ + 3e- à NO(g) + 2H2O |
0.964 |
|
AuCl4- + 3e- à Au(s) + 4Cl- |
1.001 |
|
Br2(l) + 2 e- ® 2 Br- |
1.077 |
|
O2 + 4 H+ + 4 e- ® 2 H2O |
1.229 |
|
MnO2 + 4 H+ + 2 e- ® Mn2+ + 2 H2O |
1.229 |
|
Cr2O72- + 14H+ + 6e- ® 2Cr3+ + 7 H2O |
1.33 |
|
Cl2(g) + 2 e- ® 2 Cl- |
1.360 |
|
ClO3-(aq) + 6H+(aq) + 5e- ® ½Cl(g) + 3H2O |
1.458 |
|
Au3+ + 3e- ® Au |
1.498 |
|
MnO4- + 8 H+ + 5 e- ® Mn2+ + 4 H2O |
1.512 |
|
PbO2(s) + SO42-(aq) + 4H+ + 2e- ® PbSO4(s) +2H2O |
1.687 |
|
H2O2 + 2 e- ® 2 OH- |
1.763 |
|
Co3+ + e-® Co2+ |
1.953 |
|
F2(g) + 2 H+ + 2 e- ® 2 HF(aq) |
2.889 |
Those at the top really don’t want to happen as written. Those near the bottom (most Å ) do. If you want a E0ox, switch sign
Zn ® Zn2+ + 2e- E0ox=0.762
Voltage is electrical pressure and just as with water the pressure does not depend upon how much water is present, voltage is independent of how much. So if you multiply and equation by 2, "E" it is the same! Voltage (EMF or potential difference) depends on P.E. which is determined by bonding arrangements of the atoms. This is the "Driving Force." What does depend upon how much is current?
B. Relative strengths of oxidizing and reducing agents
| Zn2+(aq) + 2e- ® Zn(s) | E0red = -0.762 V |
| 2H+(aq) + 2e- ® H2(g) | E0red = 0.000V |
| Cl2(g) + 2e- ® 2Cl-(aq) | E0red = +1.360V |
Oxidizing agents become stronger moving down left column
2. Reducing agents (right column, Table 21.1). The larger the value of E°, the stronger the reducing agent.
| Zn(s) ® Zn2+(aq) + 2e- | E0ox = +0.762 V |
| H2(g) ® 2H+(aq) + 2e- | E0ox = 0.000 V |
| Cl2(g) ® Cl2(g) + 2e- | E0ox = -1.360 V |
Reducing agents become weaker moving down right column
Any substance on the left will react with any substance on the right that is above it. Just because you write a reaction and say it goes says nothing about the rate.
C. Calculation of standard cell voltage
E0tot = E0ox + E0red
Cl2(g) + 2Br-(aq) ® 2Cl-(aq) + Br2(l)
E0tot = E0red Cl2(aq) + E0ox Br-(aq)
= 1.360 V – 1.077 V= +0.283 V
Since calculated voltage is positive, this reaction can occur in a voltaic cell.
Reread p. 743-744
D. Determination of whether redox reaction will occur when reagents are mixed in the laboratory. Reaction goes if calculated E°tot is positive.
Will a reaction occur if bromine is added to a solution of tin(II) chloride?
Do it for Daniel Cell!
Possible oxidations:
Sn2+(aq) + 2e- à Sn(s) E°ox = -0.154 V
2Cl-(aq) à Cl2(g) + 2e- E°ox = -1.360 V
Possible reductions:
Sn2+(aq) + 2e- à Sn(s) E°red = -0.141 V
Br2(l) + 2e- à 2Br-(aq) E°red = +1.077 V
Reaction that occurs:
Sn2+(aq) + Br2(l) à Sn4+(aq) + 2Br-(aq); E°tot = +0.923 V
See fig 21.5
Note: Some materials can be either oxidized or reduced: Fe2+, H2O2, H2O, Sn2+
Relation between E°tot, DG° and K
DG= -Wmax = -nFE max work out of system
DG°(kJ)= -nFE°tot = -96.5 nE°tot
DG°(J) = -RTlnK
So ln K = nFE°tot
RT
If E°tot +, DG° is -, K > 1
For Zn + Sn2+ à Sn + Zn2+
Fine ‘em! E°, DG°, K
E°tot = -(-0.762) + (-0.141) = 0.621 V
DG° = -2 x (96.5)(0.621) = -120. KJ
ln K = - DG = +120.
KJ x 1000 J/kJ
RT 8.31J/moleK(298K)
K = 1.1 x 1021
I found out what to do if K > 1099
Ln K = 250
elnK = e250 = K
Effect of concentration on voltage (EMF) (Non standard conditions)
Reaction 1 & 2 p. 749
For Daniel cell Zn + Cu2+ à Zn2+ + Cu
Q = [Zn2+]
E down as Q up
--------
[Cu2+]
From "Le Chat" as products [ ] go up we know the system would oppose this so Enet goes down (Enet < E°net)
p. 750 Nernst Equ.
aA + bB « cC + dD
The Nernst Equ. Is related to Le Chatelier’s principle in that they both relate to what happens to reaction tendencies when concentrations are changed. When E > 0 reactions are favored to go as written. However Nernst is QUANtitative, LeChat QUALitative.
Enet = E°net
- RT lnQ
nF
Q = [C]c[D]d
-----------
[A]a[B]b
Q is like equ. Expression BUT at equ. Opposing reaction rates are =; e- would flow through the wire in opposite directions at an = rate and Enet = 0 therefore Q is symbol for the word reaction "Quotient" and has the same form as the equilibrium constant, K but it applies to any reaction not just equilibrium.
DG = DG° + RT ln Q
If R = 8.31 J/mole*K ; T = 298K
F = Value of Faraday 96,485 J/volt
n = # of e- transferred
RT = 0.0257
F
Enet = E°net
– 0.0257 lnQ
n
See p. 750 bottom on Q
There are 2 conditions you can get E easily
Q = 1 => lnQ = 0 therefore E = E°
At Equ Q = K but Ecell = 0
A "Dead" battery has reached Equ – "Old chemist never die, they just reach equilibrium."
E°= RT ln K
So we have
a neat way of getting K by measuring E°
nF
Example: Fe2+ + H+ + MnO4- à Fe3+ + Mn2+
Given: T = 25°C, [Fe2+] = 0.50M
[MnO4-] = 0.100M, [H+] = 1.00M, [Mn2+] = 2x10-2M
[Fe3+] = 3 x 10-1M
Find: E°net, Enet & DG
5Fe2+ à 5Fe3+
+ 5e-
-0.77 V
8H+ + MnO4-
+ 5e- à Mn2+ + 4H2O
1.51 V
5Fe2+ + 8H+ + MnO4-
à 5Fe3+ + Mn2+ + 4H2O
+0.74 V
![]()
![]()
= 0.74 – (-0.0021) = 0.76 V
DG = -NFE = -5(96,485 J/V mole)(0.76V)
= -370 kJ/mole
Zn(s) + 2H+(aq) à Zn2+(aq) + H2(g)
Suppose Zn2+ = 1 M
H2 = 1ATM [Note ATM’s]
Fine [H+] & pH
If E measures 0.200 V [Tell me why!]
USE Nernst
E = E° - 0.0257 ln [H2][Zn2+]
n
[H+]2
So we need E°
0.762V
0.200V=0.762V-(0.0257)ln(1/[H+]2)
-0.562V=-0.0129ln(1/[H+]2)
-0.562V=0.0129ln[H+]2
ln[H+]2=-(0.562/0.0129)=-43.6
2ln[H+]=-43.6Þ ln[H+]=-21.8
[H+]=3.41x 10-10
pH = 9.468 (Note: 4 sig. figs)
Electrolytic Cells
Electrolysis: By supplying electrical energy we can force a non-spontaneous reaction to go.
If we try to pass an electric current through NaCl(s)-nothing, a good insulator, but if we melt it, ions are free to move.
A. Molten(fused) salts MX

M+ + e-® M
X- ® X + e-
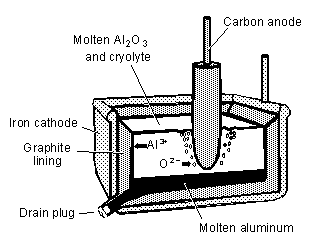
See OH(18)
http://www.scifun.chem.wisc.edu/chemweek/Aluminum/Aluminum.html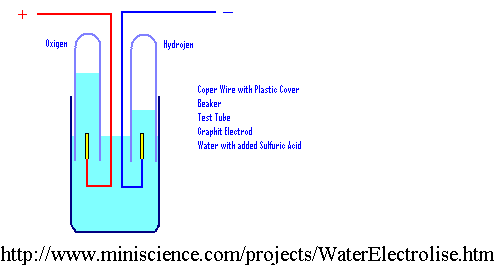
B. Electrolysis of Aqueous Solutions
1. Inert Electrodes: Here the electrodes do not enter into the reaction, but serve only as a "door" to transfer e- to the solution. We may use C or PT electrodes.To do this right, we should list ALL reactions that can occur at the electrodes. This needs to include water itself being oxidized or reduced.
Ox: 2H2O® 4e- + 4H+ +O2 E° =-1.23V
Red: 2H2O +2e-® H2 + 2OH- E° =-.83V
@Electrolysis of NaCl(aq) (IM) Do it
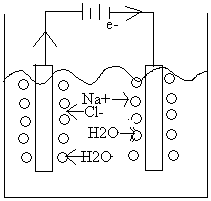 |
Write down what can happen on each electrode (there is one more factor we should look at on whether we are in acid, base, neutral…but we will ignore consideration here. |
At Anode:
2Cl-(aq) ® Cl2(g) +2e- E° = -1.36V
2H2O ® 4e- + 4H+ + O2 E° = -1.23V
At Cathode:
Na(aq) + e ® Na(g) E° = -2.71V
2H2O +2e- ® H2(g) + 2OH-(aq) E° = -.83V
We would pick water in both cases because H2O is more easily reduced and oxidized. Choose the one with the most favorable (most +, least -) E° value. BUT! WE smell Cl2 ! What’s going on?!
Note: The voltages are close to each other so both may come off. Really the evolution of O2 on graphite has a rather high overvoltage, so that Cl2(g) comes off. O2 is predicted by E° , D G but the rate is slow, mechanism considered the different between E (theoretical) and E(real) [not real, well understood]
Factors responsible for difference:
1. Ionic concentration2. Surface conditions at interface
The overvoltage for 2H+ + 2e- ® H2 is only 0.02V while 2H2O ® O2 + 4H+ + 4e- has a large overvoltage. So Cl2 wins!
So the overall reaction becomes
2Cl–(aq) ®
Cl2(g) + 2e–
–1.36 V
2H2O(l) + 2e– ®
2H2(g) + 2OH–(aq)
–0.83 V
![]()
2Cl–(aq) +2H2O(l)
® 2H2(g) + 2OH–(aq)
+ Cl2(g)
ENET = –2.19 V
So to make happen we need a minimum 2.19 V (not counting overvoltage)
Discuss, sketch what happens in the electrolysis of 1M HBr. What is E0NET?
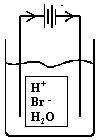
Anode: E0
2Br–(aq) ®
Br2(g) + 2e–
-1.08V
2H2O(l) ®
4e– + 4H+(aq) + O2(g)
-1.23V
Cathode:
2H+(aq) + 2e–
® H2(g)
0.00V
2H2O(l) + 2e–
® H2(g) + 2OH–(aq)
-0.83V
2Br–(aq) + 2H+(aq) ® Br2(g) + H2(g) E0NET = -1.08V
EREAL > E0NET in an absolute sense
Discuss, sketch what happens in the electrolysis of CuCl2 Solution (1M) using Graphite Electrodes
Anode: E0
2Cl–(aq) ® Cl2(g) + 2e– -1.36V
2H2O(l) ® 4e– + 4H+(aq) + O2(g) -1.23V
Cathode:
Cu+2(aq) + 2e– ® Cu(s) +0.34V
2H2O(l) + 2e– ® H2(g) + 2OH–(aq) -0.83V
Cu+2(aq) + 2Cl–(aq) ® Cl2(g) + Cu(s) E0 = -1.02V
Electrolysis involving active electrodes.
Here the electrodes themselves can undergo a reaction. Used in plating cells.
Discuss, sketch what happens in the electrolysis of CuCl2 Solution (1M) using copper electrodes.
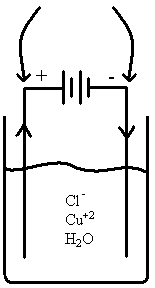 |
Anode: 2Cl–(aq) ® Cl2(g) + 2e– 2H2O(l) ® 4e– + 4H+(aq) + O2(g) Cu(s) ® Cu+2(aq) + 2e– Cathode:
|
E0 -1.36V -1.23V +0.34V
|
One electrode loses mass (anode), the other gains same mass (cathode). Atoms just transferred. If you have a mixture of metals, the one with the largest E° will plate out first.
C. QUANTATIVE ASPECTS OF ELECTROLYSIS [FARADAY’S LAW]
Faraday found the amount of chemical change that occurs in an electrolysis reaction is related to the amount of charge that goes through the system.
FARADAY= The amount of charge= to 1 mole of e-
So, 1F = 1 mole e-
1 coulomb= 1 ampere x 1 second
Experimentally* 1F = 96,500C = 1 mole e-
Charge ® Q = I x C (current x time)
C = A x S
Problem: In a copper plating cell, how many grams of Cu will be deposited from a CuSO4 solution by a current of 1.5A for 4.0 hours?
2e- + Cu+2(aq) ® Cu(s)
T = 4.0h x 60min./1hr x 60sec/1min. = 14,000 sec.
I = 1.5 A
Find: Mass of Cu
Problem: How long would it take to produce 25.0 g of Cr from a solution of CrCl3 by a current of 2.75A?
Given: Mass Cr = 25.0g; I = 2.75A
Find: Time
Cr+3 + 3e- ® Cr(s)
25.0 g Cr x (1 mole Cr/52.0 g Cr) x (3 mole e-) = 1.44 mole e-
1.44 mole e- x (96,500C/1mole e-) = 139,000C = q
139,000C x (1 AxS/1C) x (1/2.75A) = 50,500sec = 14 hours
Back to the Class Notes Homepage
 |
|