CHAPTER 20
The basic goal of chemistry is to predict whether or not a reaction will occur when reactants are brought together.
One way is if ΔH<0 reactions tend to go
SPONTANEOUSIf K>1 reactions tend to go
If ΔE is + [next chapter] reactions tend to g
oIf ΔG is –
"Nature tends to move spontaneously from a state of lower probability to one of higher probability."
G.N. Lewis – IN YOUR TEXT – p. 693
Entropy: describes the randomness involved in energy transactions. A system that is more random has a hig
her “S”. So ΔS is + means products are more random than reactants.It is a State function – See fig 20.5 and p 697, nice job in text.
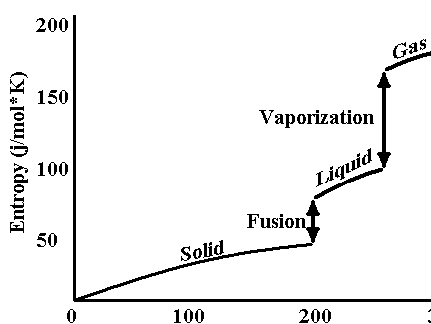 temp(K)
temp(K)
II. Entropy Changes
See table 20.1 note units. Note elements have So unlike Δ H. Why? See p.700;table 20.2
Once again, H+ Zero – Zippo – "0" – none
Note: S is + for elements and compounds.
ΔS = ΔS products –ΔS reactants
Solid à
liquid; ΔS +Liquid à
Gas; ΔS +ΔS is usually + for a reaction in which the # of moles or gas increases. See p. 701
2SO3(g) à 2SO2 (g) + O2
(g); Δng = ? so ΔS=?N2(g) + 3H2(g) à 2NH3 (
g); Δng = ? so ΔS =?ΔS
o = S So products - S So reactantsFe2O3(s) + 3H2(g) à 2Fe(s) + 3H2O(g)
ΔSo = 2SoFe(s) + 3SoH2O(g) – SoFe2O3(s) – 3SoH2(g)
= 2(27.3 J/K) + 3(188.7 J/K) – 87.4J/K – 3(130.6)
= 141.5 J/KΔ
So is nearly temp. independent (see p. 702)III. Second law of thermodynamics:H2O(s) à H2O(l) ΔS= +/- ΔH= +/-
H2O(l) à H2O(g) ΔS= +/- ΔH= +/-
At what conditions do these reactions become spontaneous?
Δ
Suniv > 0
IV. Free Energy ChangesFor spontaneous Process
- Calculation of ΔSsurr at constant T, P :
Δ
Ssurr = qsurr/TΔSsurr = -ΔH/T (constant T, P)Show by calculation that the vaporization of water at 25° C and 1 ATM is non- spontaneous
H2O(l)àH2O(g).Δ
Ssystem = SoH2O(g) – SoH2O(l) = 0.1188kJ/KΔ
Ssurr = -[ΔHf H2O(g) - ΔHf H2O(l)] / T = -44.0/298Δ
Stotal = 0.119-0.148 = -0.029 kJ/KWhere would
ΔStotal = 0?
∆G° = ∆H° - T∆S° Gibbs/Helmholtz Equation
Note that ∆G, like ∆S, is dependent on P. Unlike ∆H° and ∆S°, ∆G° is strongly temperature dependent because of T in the equation.
If ∆G° is -, reaction spontaneous at standard conditions
If ∆G° is +, reaction non-spontaneous at standard conditions
If ∆G° is 0, reaction at equilibrium at standard conditions
Free Energy: "Real" energy change not only from breaking and forming bonds (Enthalpy), but taking organization changes (Entropy) in account as well.
The striving to achieve a state of minimum free energy may be interpreted as the driving force for a chemical reaction.
Read the last paragraph p.707 & all 708
p. 709 Gives standard conditions
See p. 711 Underline
See OH #6
Comments Regarding "Standard Free Energy Changes"
The free energy of a substance (as well as its enthalpy and entropy) depends upon the state of the substance, i.e., whether it is a solid, liquid, or gas.
In addition, the free energy depends upon the temperature, the pressure, and in the case of solutions, on the concentration.
Consequently, the change in free energy that occurs during a chemical reaction depends upon the state (s, l, g), the conditions (T,P), and the concentrations of the reactants and products.
For convenience, standard conditions have been chosen for reference. These
conditions are:
| T= 25°C | P= 1 atm | Concentration = 1 M |
Standard Free Energies of Formation, ∆Gf°
Definition:
![]()
The free energy change when one mole of a substance in its free state is formed from its elements in their free states.
This definition is obviously analogous to our earlier definition on enthalpy of formation, and the calculation of the free energy change which occurs during a reaction is accomplished in a similar manner.
| Note: The superscript o, above is a thermodynamic symbol that generally indicates free or standard state. |
A QUALITATIVE LOOK at what is Implied by the Equation:
ΔG
= ΔΗ - TΔS
Since there are two possible signs for both ΔH and ΔS (positive or negative), there are four possible combinations of these signs.
If both ΔH is negative (good) and ΔS is positive (good), then ΔG must be negative, and a process for which this
is true will be spontaneous at all temperatures.If ΔH is positive (bad) and ΔS is negative (bad), then ΔG must be positive, and a process for which this is true will not be spontaneous at any temperature.
If both are + or -, then ΔG can be either negative or positive depending upon which has which sign and whether ΔH is greater or less than TΔS.
These ideas are summarized in the following chart.
Gibbs-Helmholtz equation: ΔG° = ΔH° - TΔS°
If ΔH° is +, ΔS° is -, then ΔG° is + at all T, nonspontaneous at 1 atm. *
ΔH° is -, ΔS° is +, then ΔG° is - at all T, spontaneous at 1 atm. **
ΔH° is +, ΔS° is +, then ΔG° is + at low T, becomes negative at high T
ΔH° is -, ΔS° is -, then ΔG° is – at low T, becomes positive at high T.
(*)Reverse reaction is spontaneous
(**) Reverse reaction is nonspontaneous
Practice Problem
Consider:
H2O(l) à H2O(g) at P = 1atm
If ΔH = +40.63kJ, and ΔS = +108.8 J/K·mol. What is the boiling point of H
2O(l)?ΔG = ΔH-TΔS At equilibrium, ΔG = 0, so
0 = ΔH - TΔS
ΔH = TΔS
T = ΔH/ΔS = 40630/108.8 = 373 K
* See p.715 – Equation 20.22 KNOW IT!
Practice Problem
Consider the reaction:
3/2 O2(g) à O3(g)
Calculate ΔG° for this reaction, given:
T = 25°C ΔH
f°[O3(g)] = 142.2 kJ/molS°[O2(g)] = 205.0 J/K· mol S°[O3(g)] = 237.7 J/K· mol
ΔG f° = ΔH° - TΔS°
ΔS° = 237.7 – 3/2(205.0) = -69.8 J/K
ΔG° = +142,200 – (298)(-69.8) = +163,000 J = +163 kJ
Practice Problem
Calculate the free energy change that occurs during the combustion of 6.48 grams of propane, C3H8, to form carbon dioxide and liquid water given: @25° C, 1atm
ΔG
f°[C3H8(g)] = -23.47 kJ/molΔG
f°[CO2(g)] = -394.38 kJ/molΔG
f°[H2O(l)] = -237.19 kJ/molBalanced equation is:
C3H8(g) + 5O2(g) à 3CO2(g) + 4H2O(l)
ΔG° = Σ ΔG
f°(Products) - Σ ΔGf°(Reactants)ΔG° = [3 ΔG
f°(CO2) + 4 ΔGf°(H2O)] –[ΔGf°(C3O8) + 5ΔGf°(O2)] ΔG° = [3 (-394.38) + 4 (-237.19)] –[(-23.47) + 0]ΔG° = -2108.43 kJ/mol C3H8
![]()
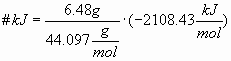
![]()
Note: normally you wouldn’t use ΔG°’s: they are very temperature dependent. Instead, use ΔH°’s and ΔS°’s.
ΔG= ΔH -TΔS
***************************************************
Calculate ΔG° at 25°C and at 50
0°C. Don’t use appendix (for ΔG°’s).![]()
![]()
![]()
![]()
![]()
D
S° = 2S° Fe
+3S° H20(g) - (S°
Fe2O3 + 3S° H2)
![]()
![]()
![]()
At
![]()
![]()
Spontaneous at 500ºC, not at 25ºC.
At what temperature does the reduction of Fe2O3 by H2 become spontaneous at 1 atm?
![]()
![]()
![]()
Relationship Between ΔG° and K
![]()
Since both the free energy change for a process and the equilibrium constant for a process describe the tendency for the process to occur, it would seem that these two quantities should be related to each other.
![]()
![]()
Read pg. 720, #1, 2, 3
| If K > 1, DG° < 0 | Reaction spontaneous at stand. cond. |
| K < 1, DG° > 0 | Reaction nonspontaneous at stand cond. |
| K = 1, DG° = 0 | Reaction at equilibrium at stand cond. |
See table 20.4 p. 720
*************************************************
Calculation of DG° for the following reaction:
H2O(l) ↔ H+(aq) + OH-(aq)
Read 721-722
What is the second law of thermodynamics?
You can’t break even!
The entropy of the universe increases!
What is the first law of thermodynamics?
Practice Problem
If the free energy of formation of HI at 490
°C is –12.1 kJ/mol, calculate K for H2(g) + I2(g) à 2HI(g)D
G = -RTln Kln K = -
DG/RTln K = -(2)(-12.1 x 103)/(8.314)(763)
ln K = +3.8149
K = e3.8149 = 45.4
Practice Problem
Consider: 2NO(g) + O2(g) à 2NO2(g)
Given: K = 1.6 x 1012 and
DGf°(NO) = +86.6 kJ/Mole at 25°CCalculate
DGf°[NO2(g)] DG° = -RTln KD
G° = -(8.314)(298)ln (1.6 x 1012)D
G° = -6.96 x 104 J = -69.6 kJ|
DG° = 2 DGf°(NO2) – 2 DGf°(NO) = |
|
D
Gf°(NO2) = [ DG° + 2 DGf°(NO)]/2D
Gf°(NO2) = [(-69.6) + 2(86.6)]/2D
Gf°(NO2) = +51.7 kJAbsolute Entropies
And
The 3rd law of Thermodnamics
The 3rd Law simply states that:
The entropy of perfect crystals of all pure elements and compounds is zero at the absolute zero of temperature.
One significant result of this law is that it allows scientists to calculate (and publish for our use) tables of absolute entropies of substances.
The calculation of absolute entropies gets rather involved. Essentially, the calculations use the relationship
D
S = nCpln T2/T1 Heat Capacity![]()
but are actually much more complicated, because Cp itself is a function of temperature, and often a very complicated one.
Fortunately, the calculations are done for us, and we simply use the published table of absolute entropies of substances.
Back to the Class Notes Homepage
 |
|