- Complex Ions and Coordination Compounds
PURDUE
has a wonderful WEBSITE to teach you this stuff.
GO THERE LEARN THE STUFF!!!!
Be sure to look on the left of this
page for more topics.
- A complex ion consists of a central metal cation (usually derived from a
transition metal) joined by coordinate covalent bonds to two or more
molecules or anions called ligands.
|
Complex ion |
Cation |
Ligands |
Coordination no. (# of bonds) |
|
Ag(NH3)2+ |
Ag+ |
2 NH3 molecules |
2 |
|
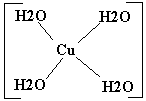
Cu(H2O)42+ |
Cu2+ |
4 H2O molecules |
4 |
|
Fe(CN)63- |
Fe3+ |
6 CN- ions |
6 |
Coordination compound contains complex ion. Examples:
[Cu(H2O)4]SO4 , [Ag(NH3)2]NO3,
K3[Fe(CN)6]
Usually Compounds, SEE OH#2
 |
Charge of central metal atom in Zn(H2O)
3(OH)+ ?
+1 = -1 + x; x = +2
Do Cu |
- Nature of ligands; ordinarily contain at least one unshared pair of
electrons.
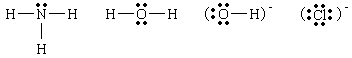
Note:
Ligands are
Lewis Bases, Metals are Lewis Acids…Look Up Why.
If the ligand contains two or more unshared pairs on different,
nonadjacent atoms, it can act as a chelating agent, forming more than
one bond with the central metal atom.
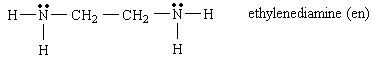
Forms bidentate complexes such as Co (en)33+
SEE OH#3
- Nomenclatures: see rules P. 553
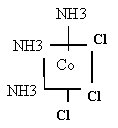
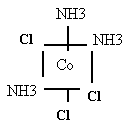
- Cation Co(NH3)4Cl+2
You have four rules- Do it, name me!
Tetra
amminedichloro
cobalt (II)
Do it, name me! Ni(en)32+
Tris
(Ethylenediamine)
Nickel (II)
Anions® Put "Ate" after metal,
name the rest same.
Do it, name me! Al(OH)4
Tetra
hydroxoaluminate
(II)
Write the formula for Pentachlorohydroxoferrate (III)
Compounds ® just like ionic compounds. Above
2 with K+
[See notes for today- Go over lab Co Comp.]
- Geometry of Complex Ions
- Coordination no. = 2; linear
180° bond angle
Name
these! Last chapter’s lab
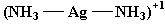
- Coordination number = 4
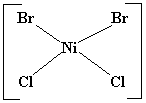
- Tetrahedral: Zn(NH3)42+
(Last Chapter’s lab) CoCl42-
| 2. Square planar: Cu(H2O)42+ |
  |
Can
show isomerism:
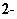
|
cis isomer
(like groups close)
polar
|
trans isomer
(like groups far apart)
nonpolar
|
- Geometry of Complex Ions
Coordination number = 6; octahedral
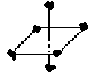 All ligands same distance from
metal atom.
All ligands same distance from
metal atom.
Any position is trans to one position and cis to four others.
See fig 16.5
Co(NH3)4Cl2+ two isomers à
see Fig 16.6, &. 7 note names
Co(NH3)3Cl3 two isomers à
see Fig 16.8
facial triamminetrichlorocobalt (III)
?
See 16.9 figure
II. Electronic Structure (Valence bond model) See p. 560 READ IT!
Electron pairs contributed by ligands enter hybrid orbitals of central metal
ion.
- Orbitals occupied by ligand pairs
Coord. No. = 2 sp
Coord. No. = 4 sp3 (tetrahedral) or dsp2
(square planar)
Coord. No. = 6 d2sp3(inner) or sp3d2(outer)
see p.561
table 16.4 see OH #7
- Procedure: apply to Ni(CN)42-,
Cr(H20)63+ (2 examples)
Put a line down the center of your page [follow page 562 the rules]
1. Determine electron configuration of central metal.
(no s electrons)
Ni2+ 3d8
Cr3+ 3d3
2. Determine coordination number: 4, 6
3. Decide upon hybridization: dsp2
see p 562 rule "a" to find it is
d2sp3
4. Locate electron pairs in hybrid orbitals
5. Distribute electrons of metal
in accordance with Hund’s rule.
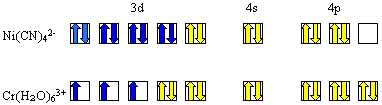
Bottom one is paramagnetic
III. Equilibria Involved in Complex Ion Formation
- Formation constants
Ag+(aq) + 2NH3(aq) ß
> Ag(NH3)2+(aq); Kf
= 2 x 107
Ag+(aq) + 2CN-(aq) ß
> Ag(CN)2-(aq); Kf = 1 x 1021
Ag(CN)2- more stable than Ag(NH3)2+
see OH #11
- Application: Calculate ratio of [Ag+]/[Ag(NH3)2+]
in 0.1 M NH3
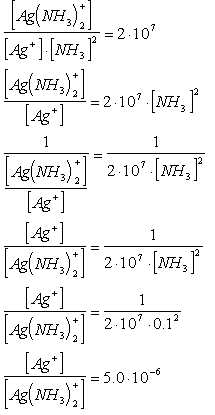
This shows most of the silver is complexed.
|
Coordination # |
Choices |
How to Choose |
Hybridization |
Geometry |
|
2 |
sp |
-------- |
sp |
Linear |
|
4 |
sp3, dsp2 |
Told geometry or hybridization |
sp3 |
tetrahedral |
|
Told geometry or hybridization |
dsp2 |
square planar |
|
6 |
d2sp3, sp3d2 |
d0-d3 |
d2sp3 |
octahedral |
|
d4-d7 low
spin |
d2sp3 |
octahedral |
|
d4-d7 high spin |
sp3d2 |
octahedral |
|
d8-d10 |
sp3d2 |
octahedral |
For the test you should be able to do this type of problem:
Co (en) Cl4- (high spin)
Name this ion.
Using V.B. Model Deduce
The electron configuration.
The coordination #.
The hybridization.
Draw the orbital diagram.
Show Geometric isomers! Draw them, if any.
¬ tetrachloroethylenediaminecobaltate
(III)
[Ar] 3d6
® Coordination # = 6
¯
sp3d2
3d
4s
4p
° (¯)(
)(
)(
)(
)
(¯) (¯)(¯)(¯)
4d
(¯)(¯)(
)(
)(
)
| ± |
 |
Back to the Class Notes Homepage
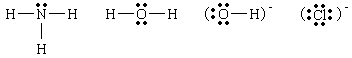
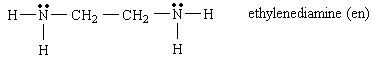



 All ligands same distance from
metal atom.
All ligands same distance from
metal atom.