 |
Acid Anhydride: a nonmetal oxide that reacts with water to form an acid (acid rain). |
Chapter 14
Operational Definition of an Acid
Acids are substances that have the following properties:
Operational Definition of a Base
Bases are substances that have the following properties:
See p.469 Reactions #1&2
Svante Arrhenius-1884
Proposed first "correct" theory of acids and
bases that is still being used.
Arrhenius Definition of Acids and Bases
Acid – A substance that produces H+ in aqueous solution (or that increases the H+ concentration in an aqueous solution).
Base – A substance that produces OH- in aqueous solution (or that increases the OH- concentration in an aqueous solution).
Types of Substance that are Arrhenius Acids
"Classical" Acids:HCl, HNO3, H2SO4, HClO4, HBr, HI, HC2H3O2, HF, HSO4-, H3PO4, H3PO3, H2CO3, etc.
Nonmetallic oxides:
CO2, SO2, SO3, Cl2O7, NO2, P2O3, P2O5, etc.
 |
Acid Anhydride: a nonmetal oxide that reacts with water to form an acid (acid rain). |
H2O + SO3 ® H2SO4 Strong acid
H2O + CO2 « H2CO3 Weak acid
Certain metallic ions, notably those that have a relative high charge and/or, a small radius:
Al3+, Cr3+, Fe3+, and some +2 ions (to a lesser extent).
ALSO
NH4+
Types of Substance that are Arrhenius Bases
"Classical" Bases:
NaOH, KOH, Ca(OH)2, NH4OH
Ammonia and other organic amines:
NH3, CH3NH2 (methyl amine), C6H5NH2 (aniline), C5H5N (pyridine), N2H4 (hydrazine), etc.
Metallic Oxides:
BaO, MgO, Na2O, K2O, etc.
|
|
Basic Anhydride! A metal oxide that reacts with water to form a base. |
BaO + H2O ® Ba(OH) 2
Na2O + H2O ® 2NaOH
Substances Containing Anions from Weak Acids:
Na2S, KC2H3O2, NaHCO3, NaF, NaCN, etc.
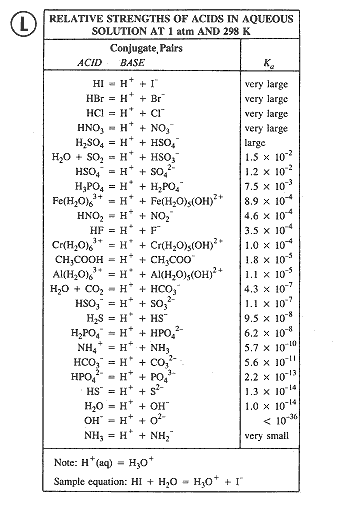
Strong vs. Weaks Acids
Common misuses of the term "strong":
Used to mean highly corrosive
As a synonym for concentrated
Common Strong Acids and their approximate Ka Values:
In order of decreasing strength
HI----------1011
HBr--------109
HClO4-----107
Know these (not #’s,
HCl--------107
but which are strong)
H2SO4-----102
See P. 475
HNO3------20
Note: For practical purposes, all these acids are equally strong in water, i.e., 100% ionized. To distinguish their relative strengths, we must use a "differentiating" solvent, such as diethyl ether.
Reread p.400-404, Ex. 3
Weak Acids
The best method of comparing the relative strengths of weak acids is to compare the relative values of their ionization constants, Ka.
Weak Acids: HA « H+ + A-
HF(aq) « H+(aq) + F-(aq)
HSO4-(aq) « H+(aq) + SO42-(aq)
NH4+(aq) « H+(aq) + NH3(aq)
Zn(H2O) 42+(aq) « H+(aq) + Zn(H2O)3(OH) + (aq)
Al(H2O)63+(aq) « H+(aq) + Al(H2O)5OH2+(aq)
M(H2O)4 or 6x+(aq) « H+(aq) + M(H2O)3 or 5OH(x-1)+(aq)
Common Name Formula Constant pKa acetic acid CH3COOH 1.75 x 10-5 4.756 ammonium ion NH4+ 5.60 x 10-10 9.252 anilinium ion C6H5NH3+ 2.54 x 10-5 4.596* benzoic acid C6H5COOH 6.31 x 10-5 4.200* chloroacetic acid CH2ClCOOH 1.36 x 10-3 2.866* cyanic acid HOCN 3.54 x 10-4 3.451 dichloroacetic acid CHCl2COOH 5.68 x 10-2 1.246* dimethylammonium ion (H3C)2NH2+ 1.82 x 10-11 10.739 ethanol CH3CH2OH 1.31 x 10-14 13.882 formic acid HCOOH 1.86 x 10-4 3.732 hydrazoic acid HN3 2.37 x 10-5 4.625 hydrazinium ion H2NNH3+ 1.03 x 10-8 7.989 hydrochloric acid HCl greater than 1 negative hydrocyanic acid HCN 5.85 x 10-10 9.233 hydrofluoric acid HF 6.94 x 10-4 3.159 hydroxylammonium ion HONH3+ 1.12 x 10-6 5.951* hypochlorous acid HClO 2.81 x 10-8 7.551 imidazolium ion C3H3NNH2+ 1.02 x 10-7 6.992* lactic acid H3CCHOHCOOH 1.37 x 10-4 3.863 methylammonium ion H3CNH3+ 2.39 x 10-11 10.622 ethanolammonium ion HOCH2CH2NH3+ 3.17 x 10-10 9.499* nitric acid HNO3 27.79 -1.444* nitrous acid HNO2 5.98 x 10-4 3.224 perchloric acid HClO4 38.29 -1.583* phenol C6H5OH 1.08 x 10-10 9.968* pyridinium ion C5H5NH+ 6.80 x 10-6 5.167* trimethylammonium ion (H3C)3NH+ 1.72 x 10-10 9.763 water H2O 1.01 x 10-14 13.994
Common Name Formula Constant pKa arsenic acid H3AsO4 K1 = 5.65 x 10-3 2.248 - H2AsO4- K2 = 1.75 x 10-7 6.757 - HAsO42- K3 = 2.54 x 10-12 11.596 boric acid H3BO3 K1 = 5.78 x 10-10 9.238 carbonic acid "H2CO3" K1 = 4.35 x 10-7 6.361 - HCO3- K2 = 4.69 x 10-11 10.329 chromic acid H2CrO4 K1 = 3.55 -0.550* - HCrO4- K2 = 3.36 x 10-7 6.473* citric acid HOC(CH2COOH)3 K1 = 7.42 x 10-4 3.130* - - K2 = 1.75 x 10-5 4.757* - - K3 = 3.99 x 10-6 5.602* EDTA C2H4N2(CH2COOH)4 K1 = 9.81 x 10-3 2.008* - - K2 = 2.08 x 10-3 2.683* - - K3 = 7.98 x 10-7 6.098* - - K4 = 6.60 x 10-11 10.181* glycinium ion H3NCH2COOH+ K1 = 4.47 x 10-3 2.350* -(glycine) H2NCH2COOH K2 = 1.67 x 10-10 9.778 hydrogen sulfide H2S K1 = 1.02 x 10-7 6.992 - HS- K2 = 1.22 x 10-13 12.915 oxalic acid HOOCCOOH K1 = 5.40 x 10-2 1.268* - HOOCCOO- K2 = 5.23 x 10-5 4.282 phthalic acid C6H4(COOH)2 K1 = 1.13 x 10-3 2.946* - - K2 = 3.90 x 10-6 5.409* phosphoric acid H3PO4 K1 = 7.11 x 10-3 2.148 - H2PO4- K2 = 6.23 x 10-8 7.206 - HPO42- K3 = 4.55 x 10-13 12.342 succinic acid C(CH2)2COOH K1 = 6.21 x 10-5 4.207* - HOOC(CH2)2COO- K2 = 2.31 x 10-6 5.636* sulfuric acid H2SO4 K1 > 1 negative - HSO4- K2 = 1.01 x 10-2 1.994 sulfurous acid H2SO3 K1 = 1.71 x 10-2 1.766 - HSO3- K2 = 5.98 x 10-8 7.223
Very Important** HA « H+ + A-
Ka = [H+][A-]
pKa
= -logKa
Autoionization of Water
Water is very slightly ionized in to H+ and OH- ions.
H2O « H+ + OH-
2H2O « H3O+ + OH-
Nature of the "H+" ion – preferably written as H3O+ (hydronium ion)
Actual species is probably closer to H9O4+
Ion Product of Water, Kw
Look up in your text Amphoteric and Amphiprotic. Use index.
pH and pOH
In 1909, a Danish biochemist, Soren Sorensen, defined a quantity called the "pH of a solution." It was defined as
pH = -log[H+]
What good is this? Why would we want to have such a definition?
Finding the pH of pure water:
(x)(x) = 1.0´ 10-14
pH = -log 1.0´ 10-7 M = -(-7) = 7
By a similar process, we can define the pOH of a solution, or pKa of a weak acid, etc.
Relationship between pH and pOH
Since:
[H+] [OH-] = 1.0´ 10-14
log [H+] + log [OH-] = log(1.0´ 10-14) = -14
-log [H+] – log [OH-] = 14
pH + pOH = 14
What range can pH (and pOH) values assume?
On a blackboard----anything.
In reality, from about –1 to 15.
Neutral solution: pH = 7.0
Acidic solution: pH < 7.0
Basic solution: pH > 7.0
Suppose [H+] = 2.4´ 10-6 M; calculate pH
pH = -log10 (2.4´ 10-6) = 5.62
Suppose pH = 8.68; calculate [H+]
Use 10x or "–" then INV and LOG keys;
[H+] = 10-8.68 = 2.1´ 10-9 M
pOH = -log10 [OH-] ; pOH + pH = 14.00
What is [H2O]?
Strong Acid
Practice Problem
Calculate the concentrations of H3O+
, HCl (molecules), OH- , Cl- , the pH, and the pOH of a 0.00800 M solution of HCl.Note: On this and all subsequent problems, it will be assumed that the temperature is 25.0oC unless stated otherwise.
Assumptions:
Since HCl is a strong acid (100% ionized), it is assumed that virtually all the H+ in the solution comes from the HCl, and the amount coming form the water can be neglected.
HCl ® (100%) H+ + Cl-
INT 0.00800 M 0 0
D -0.00800 M 0.00800 M 0.00800 M
EQU 0 0.00800 M 0.00800 M
PH = -Log [H+] = -Log 0.00800 = 2.097 NOTE sig Figs
POH = 14 – pH = 14.000 – 2.097 = 11.903
[OH-] = 10-pOH = 10-11.903 = 1.25´ 10-12 M
Strong Base
Practice Problem
Calculate the concentrations of Ba2+, OH-, and the pH of a 0.0200 M solution of Ba(OH)2.
Strong Base – How do you know?
Ba(OH)2 ® (100%) Ba2+ + 2OH-
INI 0.0200 M 0 0
D -0.0200 M 0.0200 M 0.0400 M
EQU 0 0.0200 M 0.0400 M
[OH-] = 0.0400 M
[H+][OH-] = 1.0´ 10-14
[H+] = (1.0´ 10-14/[OH-]) = (1.0´ 10-14/0.0400) = 2.5´ 10-13 M
pH = -Log [H+] = 12.60
Again NOTE the sig Figs
Variation of Kw With Temperature
Since the ionization of water is an equilibrium process, the extent of ionization, and thus the value of the equilibrium constant for the process, Kw, will vary with temperature.
The ionization of water is an endothermic process.
H2O « H+ + OH-
DH = +55.9 kJAccording to Le Chatelier’s Principle, if temperature is increased, the system should shift to the right in an attempt to try and lower the temperature. This would result in higher concentrations of both H+ and OH-, so Kw should
increase with temperature.Temperature ° C Kw
0 0.114´ 10-1410 0.292´ 10-14
20 0.681´ 10-14
25 1.01´ 10-14
30 1.47´ 10-14
40 2.92´ 10-14
50 5.47´ 10-14
60 9.61´ 10-14
100 5.5´ 10-13
Weak Acid
Practice Problem
Calculate the concentrations of H3O+, OH-, F-, HF-, the pH, pKa, and the % ionization of a 0.175 M solution of HF.
Ka for HF = 3.5´ 10-4
HF « H+ + F-
INI 0.175 M 0 0
D -x x x
EQU 0.175 - x x x
Ka = [H+][F-] =
(x)(x) = 3.5´
10-4
--------------
----------
If we want to, we can solve this quadratic equation for ‘x’.
However, since HF is a weak acid (we know this because Ka is
small), ‘x’ (the amount of HF ionized) must be a "small" number.
If this is true, then 0.175-‘x’ would be approximately equal to 0.175, and
we might be able to "neglect ‘x’". Let’s try this assumption and
see what we obtain for an answer.
(x)(x) = 3.5´ 10-4
----------------
0.175 - x
x2 = 6.125´ 10-5
x = 7.83´ 10-3 (2 sig figs allowed)But is this negligible?
It depends upon your standards.
Most textbooks suggest that you can "neglect ‘x’" if it is less that 5% of the number it was subtracted from (or added to). The logic behind this is that when we substitute molarities into an equilibrium constant expression, we are only approximating the actual numbers, which should be utilized anyway. We really should be using "activities," not molarities, in the first place. If we accept this proposal, we now can see if our assumption that ‘x’ was negligible was valid.
7.83´ 20-3 ´
100 = 4.47%
-------------
0.175
Note: This is also the calculation of % ionization.
In this problem, we just made it!
Therefore:
[H+] = [H3O+] = [F-] = x = 7.8´ 10-3 M
[OH-] = 1.0´ 10-14 = 1.0´
10-14 = 1.3´ 10-12 M
------------- -------------
[HF] = 0.175 - x = 0.175 – 0.0078 = 0.167 M
pH = -log(7.8´ 10-3) = 2.11
pKa = -log Ka = 3.46
More Comment on the "Is ‘x’ Negligible?" Decision
What do you do if ‘x’ isn’t negligible?
Basically, you have two choices.
Example of how this works—look at the last problem.
x1 = 7.8262´ 10-3
(x2)(x2)
= 3.5´ 10-4
------------------------
0.175 - 0.0078262
x2 = 0.0076492
(x3)(x3)
= 3.5´ 10-4
---------------------------
0.175 - 0.0076492
x3 = 0.0076533, Etc.
Practice Problem
The pH of a 0.345 M solution of a weak acid, HA, is 4.14. Calculate the ionization constant for this acid.
HA « H+ + A-
INI 0.345 M 0 0
D
EQU
Ka = [H+][A-]
These are at equilibrium
-----------
[HA]
I know pH so I can get [H+] at equilibrium
pH = 4.14, Then [H+] = 10-4.14 = 7.2´ 10-5
So [A-] = 7.2´ 10-5 M
[HA] = 0.345 – 0.000072 » 0.345
Ka = (7.2´ 10-5)2
= 1.5´ 10-8
-------------------
% Ionization = 7.2´ 10-5 ´
100%
------------
0.345
Polyprotic Acids
Two important things to remember are the ionization of polyprotic acids.
Stepwise ionization of H2 SO4
H2 SO4 ® H+ + HSO4- K1 = very large
HSO4- « H+ + SO42- K2 = 1.2´ 10-2
Stepwise ionization of H3PO4
H3PO4 « H+ + H2 SO4- K1 = 7.52´ 10-3
H2PO4- « H+ + HPO42- K2 = 6.23´ 10-8
HPO42- « H+ + PO43- K3 = 2.2´ 10-13
Weak Bases
NH3(aq) + H2O « NH4+(aq) + OH-(aq)
-(aq) + H2O « HF(aq) + OH-(aq) B. Dissociation constantF
NH3: Kb = [NH4+] ´ [OH
Kb ´ Ka = [HB] ´ [OH-] ´ [H+] ´ [B
-] = [H+] ´ [OH-] = Kw|
Ka |
Kb |
||
|
HF |
6.9 x 10-4 |
F- |
1.4 x 10-11 |
|
HC2H3O2 |
1.8 x 10-5 |
C2H3O2- |
5.6 x 10-10 |
|
NH4+ |
5.6 x 10-10 |
NH3 |
1.8 x 10-5 |
Strength of base is inversely related to that of conjugate weak acid.
See Page 5½ (My 8)
NaF
® Na+ + F-F- + H20 « HF + OH-
INI 0.10 M 0 0
D
-x x xEQU 0.10 – x x x
Kb = 1.4 ´ 10-11 from above table
Kb = [HF] ´ [OH-] = 1.4 ´
10-11 = x2 =
x2
----------------------
-------- ---------
[F-]
0.10 – x 0.10
So x = 1.2 x 10-6 M
and [H+] = 8.0 ´ 10-9
Salts that Produce Acidic or Basic Solutions-Hydrolysis
Although we usually tend to think of salts as producing "neutral" solutions, many salts, when dissolved in water, actually produce a solution which is acidic or basic.
NaCl ------------ neutral
KNO3 ----------- neutral
NaC2H3O2 ------ basic
KF --------------- basic
NaHSO4 -------- acidic
NH4Cl ---------- acidic
NaHCO3 -------- basic
Na2S ------------ basic
Al(NO3)3 ------- acidic
CaCl2 ----------- neutral
KCl ------------- neutral
See p. 488 – 493
Lecture 3
Acid-Base Properties Salt Solutions
Must consider effect of cation and anion separately. Then combine these effects to give overall result for salt. P.493
Li+, Na+, K+, Ca2+, Sr2+, Ba2+
Cl-, Br-, I-, NO3-, ClO4-, SO42-
Salt Cation Anion NaNO3 Na+ (N) NO3- (N) neutral KF K+ (N) F- (B) basic FeCl3 Fe3+ (A) Cl- (N) acidic
See p. 493 Table 14.7- really know this.
What if both cation and anion hydrolyze (react with water). You can predict whether the solution is acid or base by comparing values of Ka and Kb.
Example:
Will a solution of Al2(CO3)3 be acidic or basic?
The two reactions which can occur are:
Al(H2O)63+ + H2O « Al(H2O)5OH2+ + H3O+
Or Al(H2O)63+ « Al(H2O)5(OH-)2+ + H+
And CO32- + H2O « HCO3- + OH-
You need to look up the Ka of the acid and the Kb of the base. The large value wins!
The Formation of Salts from Acids and Bases
Neutral, Salt from a strong acid and a strong base:
![]() NaCl
Strong Acid HCl
NaCl
Strong Acid HCl
Strong Base NaOH
![]()
Base, Salt from a weak acid and a strong base:
![]() NaC2H3O3 Weak Acid
HC2H3O3
NaC2H3O3 Weak Acid
HC2H3O3
Strong Base NaOH
![]()
Acid, Salt from a strong acid and a weak base:
![]() NH4Cl
Strong Acid HCl
NH4Cl
Strong Acid HCl
Weak Base NH4OH
![]()
?????? Salt of a weak acid and weak base
![]() (NH4)2CO3 Weak Base
CO32-
(NH4)2CO3 Weak Base
CO32-
![]()
Weak Acid NH4+
Practice Problem
Calculate the pH of a 0.100 M solution of NaC2H3O2.
Reaction involved is:
C2H3O2– + H2O « HC2H2O2 + OH–
How to determine Kb C2H3O3–
if it isn’t given.
The hydrolysis or "base" constant for the acetate ion is going to
be related to the ionization constant, Ka, of its conjugate acid,
namely acetic acid, HC2H3O2.
The weaker the acid is (smaller Ka), the stronger the base must be (larger Kb).
Ka ´ Kb = Kw
The mathematical relationship between these two quantities is given by the equation:
Kb= Kw/Ka
Derivation of this relationship:
H2O « H+ + OH- K= Kw
C2H3O2- + H+ « HC2H3O2 K= 1/Ka
C2H3O2- + H2O « HC2H3O2 + OH- Kb = Kw/Ka
So Kb = 1.0´ 10-14 = 5.68´
10-10
-----------------
1.8´ 10-14
Practice Problem—Actual Solution
Calculate the pH of a 0.100 M solution of NaC2H3O2.
Always put an equation:
NaC2H3O2 ® Na+ + C2H3O2– Goes 100%
C2H3O2– + H20 « HC2H3O2– + OH– Does not go 100%
We want pH ![]() Need H+, get from OH–
Need H+, get from OH–
We can look up Kb or get from Ka
C2H3O2– + H20 « HC2H3O2– + OH-
INI 0.100 M Who cares 0 0
D
-x | x xEQU 0.100 – x ¯ x x
Kb = [HC2H3O2] ´
[OH-] = 5.68´ 10-10
= x2
----------------------------------
-------------
[C2H3O2-]
0.100 - x
Assume ‘x’ is small [put this on test] compared to 0.100
x2 = 5.68´ 10-11
x = [OH-] = 7.54´ 10-6pOH = -log [OH-] = -log 7.54´ 10-6 = 5.123
pH = 14.00 – 5.123 = 8.877
Bronsted-Lowry Definition of Acids and Bases
Is the second of the really important and popular definition of acids and bases.
Was proposed independently in 1923 by Tomas Martin Lowry in England and Johannes Nicolas Bronsted in Denmark. Bronsted really did the better work on the concept, so sometimes this is just referred to as the Bronsted definition.
Definition
Acid – a proton donor
Base – a proton acceptor
Differences from the Arrhenius Definition
Such substances are referred to as being amphiprotic.
Actually, virtually any substance containing protons could be amphiprotic under appropriate conditions. The term usually is used to refer to substances which can either donate or accept protons under "normal" conditions in aqueous solution such as HCO3- (but not HSO4-), H2PO4-, HC2O4-, HS-, or even water itself.
When a B-L acid reacts with a B-L base, the products are another acid and another base, usually referred to as the conjugate acid and the conjugate base. The resulting solution (assuming the reaction takes place in solution) is not necessarily neutral. It can be anything. The only relevant question is to what extent does the reaction proceed.
You decide by considering the relative strengths of the two acid involved in the reaction (or the two bases). Essentially, a stronger acid and base are always converted into a weaker acid and base.
HF(aq) + OH-(aq) « F-(aq) + H2O
acid base
NH3(aq) + H2O « NH4+(aq) + OH-(aq)
base acid
HCl(aq) + H2O « H3O+(aq) + Cl-(aq)
acid base
H3O+ is referred to as the conjugate acid of H2O
OH- is the conjugate base of H2O
Amphiprotic: Act as either an acid or a base: H2O, HCO3-, H2PO4- can donate or accept an H+.
Amphoteric: Can react with both H+ and OH-. Al(OH)3, Zn(OH)2, Al.
Remember complex ion in the lab where both Acid and Base caused these to dissolve.
Lewis Definition of Acids and Bases (1923)
Acid – An electron pair acceptor (Electrophilic)
Base – An electron pair donor (Nucleophilic)
Examples of Lewis Acids:
BF3, Al3+, Ag+, Zn2+, Cr3+, Ti4+, and many others.
Examples of Lewis Bases:
NH3, H2O, Co32-, OH-, F-, and many others.
Lewis Acid-Base Reactions:
All Lewis acid-base reactions can be described as conjugation—two species simple bond together with what is referred to as a coordinate covalent bond.
"Classic" example:
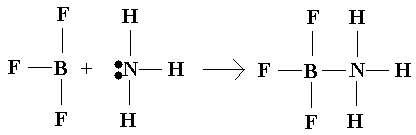 BF3
+ NH3 ® F3BNH3
BF3
+ NH3 ® F3BNH3
H+(aq) + H2O ® H3O+(aq)
acid base
Back to the Class Notes Homepage

Maintained by: Chris Weilemann
Lee Marek