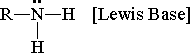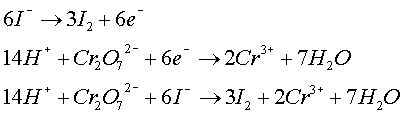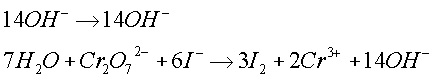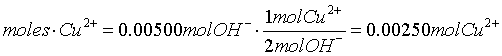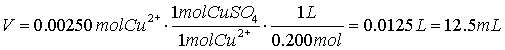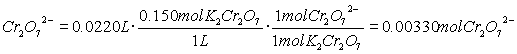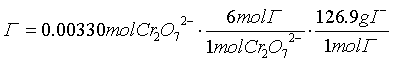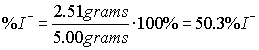- What is an acid?
- What is a base?
- Acid-Base Reactions
Forms H+ ions in H2O
What are two types? What is the difference?
Strong – 100% ionization ß text is wrong
Weak – Partial ionization
What are the classic strong acids?
(OH – 2A)
What are the classic weak acids?
See page 400 Table 12.2
(hint: Know the strong ones, and the others are weak)
1 M HCl is all H+ and Cl-
1 M HF is [HF] = 0.97 M, [H+] = [F-] = 0.03
Something that forms OH- in H2O
There are strong and weak bases. ß know the strong ones
What is a classic weak base?
What is an amine?
[CH Junk] R-NH2 + H2O ßà RNH3+ + OH-