 Click
on the picture to enlarge.
Click
on the picture to enlarge.
[see chapter #2, page 22] à see overhead #3.
The breaking apart of an ionic compound into its ions
NaCl(S) ® Na(aq)+ + Cl(aq)-
[2 moles ions]Ca(NO3)2(s) ® Ca(aq)2+ + 2NO3(aq)-
[3 moles ions]
- [see fig. 11.4]; overhead #4.
The breaking apart of covalent compounds to form ions.
HA(aq) « H(aq)+ + A(aq)-
HC2H3O2(aq) « H(aq)+ + C2H3O2 (aq)-
- Dissolve as molecules
C2H5OH(l) ® C2H5OH(aq)
C12H22O11(s) ® C12H22O11(aq)
-See tables P. 358 + P. 362
PPm (liq. sol.) = Mass % x 10 (Not in % 106)
PPb (liq. sol.) = Mass % x 10
XA = ___________
Dissolve 12.0g CH3OH in 100.0g H2O. . .
What is the mole fraction of methanol?
What is the mole fraction of water?
m = ____________
What is the molality of CH3OH?
M = ____________
 Click
on the picture to enlarge.
Click
on the picture to enlarge.(Solution d = 1.012 g/mL)
-work with 1L of solution
Mass = 1000mL x 1.012 g/mL = 1012g
Mass Al(NO3)3 = 0.200mol x 213.0g/mol = 42.6g
Mass H2O = 1012g – 43g = 969g
Therefore. . . m = 0.200mol/0.969kg = 0.206m
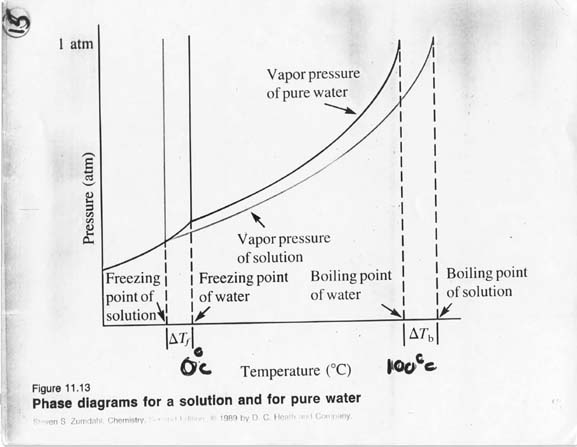
--see figure 11.11 on page 377—
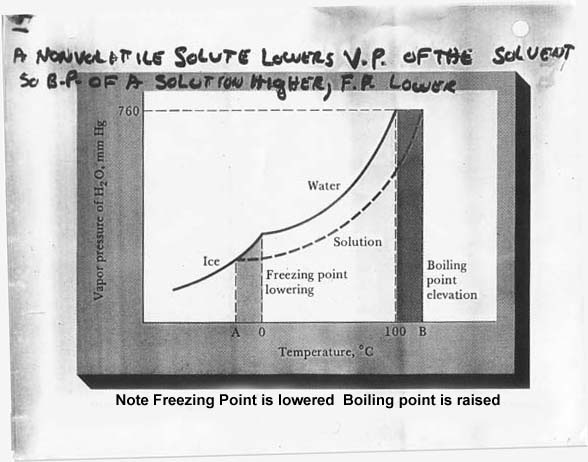
---see figure 11.15 on pg. 380—A non-volatile solute lower vapor pressure of the solvent; therefore, boiling point of a solution rises, freezing point lowers.
: F.P. depression, B.P. elevation, V.P. lowered, and as osmotic pressure of electrolytes are higher for the same concentration than non-electrolytes due to dissociation.
See Figure 11.7—it may help!
-Most non-electrolytes that are appreciably soluble in water are H-bonded [CH3OH, NH3, Sugars]. Non-electrolytes are often more soluble in non-polar solvents like CCl4 or Benzene (C6H6).
Click here to see what solutions form...

-As the temperature goes up, endothermic is favored.
D H + solid + water « solution
-The D H is usually positive so solubility goes up as temperature goes up.
-Solubility can be in (g. of solute)/(100 g solvent) or M.
gas + water ® solution + D H
-D H is usually negative, so solubility goes up as temperature goes down.
 click
on this picture to view the diagram from your book
click
on this picture to view the diagram from your book

-Negligible except for gases:
Henry’s Law Þ C(g) = kPS
Solubility = Constant x Particle Pressure
To see this effect, go to my website
-Non-electrolytes depend primarily upon the concentration of solute and the number of particles in the solution.
Vapor Pressure (V.P.) Lowering
-Raoult’s Law: vapor pressure of each substance in a solution is "a " the mole fraction of that substance.
P1 = x1P1°
P1 = Particle pressure of substance1 in solution
P1° = V.P. of pure substance1
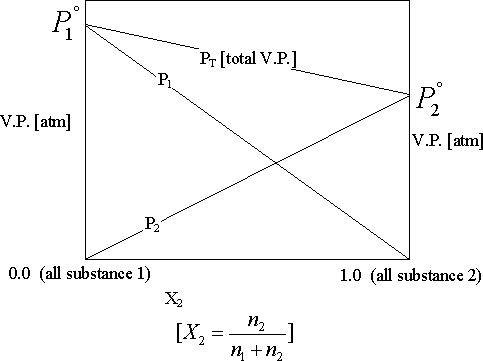
-When P2° = 0, as when a non-volatile solute [like C6H2O2 or NaCl] is added to a volatile solvent, some of the molecules on the surface layer are replaced by non-volatile solute molecules; so the V.P. is lowered. One can use this to determine the molecular weight of a substance.
V.P. Lowered = x2 P1° [See equation 11.11]
See demo below, at the end of this web page or
Figure 11.11 in your text.
See Chap. 13
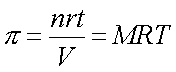
See Figure 11.15 below on this web page
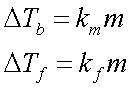 See Table
11.4
See Table
11.4
See page 384.
The higher the degree of dissociation the more the solution affected. We can use the above colligative properties as a measure of dissociation.
i º (D P)/(D P)0 = (D Tb)/(D Tb)0 = (D Tf)/(D Tf)0 = p /p 0
See pg. 385 kmThe F.P. of a 0.010m solution of HF is -0.024° C. Determine the extent HF ionizes (find ‘i’ & % ionization).
HF « H+ + F-, D T REAL = k*m*i
i = D Tf)/(D Tf)0 = (D Ts)/(kfm)
= (0.024)/(1.86 x 0.010)
= 1.3 ß 30% ionization
1. Ionic crystals dissociate in water and yield solutions of ions.
2. Strong interionic attractions between ions in solution result in a clustering action which decreases the effective concentration of individual ions.
3. The interionic attractive forces decrease as the solution is diluted because the ions are farther apart. (Table 11.5).
4. The magnitude of the interionic attraction and the degree of clustering actions are related to the charge on the ions. (Table 11.5).
Be able to tell me a STORY!!!!
 Chickens
(
Chickens
(
Ca2+
CO2(g) «
CO2(aq) «
H2CO3(aq)- «
H+ + HCO3(aq)
« H+
+ CO3
(aq)2+ «
CaCO3 (s)
(chicken breath)
(egg shell)
When the chicken pants, the equilibrium is perturbed by the rapid loss of carbon dioxide/ Because this affect cascades through all of the equilibria, the effect is a loss of solid calcium carbonate which ultimately produces weaker egg shells.
-Ted Odom, while a graduate student at the University of Illinois, found the deceptively simple "solution" to this problem—give the chickens carbonated water. Not the equilibrium has been perturbed in the opposite direction. The addition of aqueous carbon dioxide shifts all of the equilibria to the right and results in stronger egg shells. Moreover, the chickens seem to like the carbonated water, and there are rumors that they spend their spare time singing familiar jungles about "spirit" and "the real thing." Philosophical questions about which came first are left to the reader, but in this case, at least, Le Chatelier’s Principle comes before the egg (shell).
--David B. Brown, University of Vermont and John A. MacKay III, Davidson College
-When eating hot peppers or hot spicy foods has left you "breathing fire" drinking water will not relieve you, because the water cannot dissolve the oils which give the spicy taste. A chemist might suggest that the ideal would be to drink a chaser of a non-polar liquid. But most such liquids are toxic, and even gargling with benzene or toluene doesn’t sound very pleasant. A better solution is to eat greasy foods like meats which will dissolve the oils. Alternatively, food like pasta or bread, that can absorb the oils will help "put out the fire." The smell and taste of onions and garlic are also due to oils that are not easily washed away by water and are not absorbed well
Back to the Class Notes Homepage
 |
|