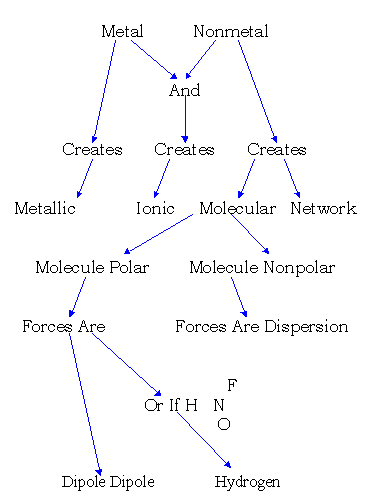
Structures and Properties of Types of Substances
| Type |
Structural Units |
Forces Within Units |
Forces Between Units |
Properties |
Examples |
|
Molecular |
Molecules: Nonpolar |
Covalent Bond |
Dispersion |
Low mp, bp; often gas or liquid at 25°C. Nonconductors. Insoluble in water, soluble in organic solvents. |
H2
CCl4 |
|
Polar |
Covalent Bond |
Dispersion, Dipole, H Bonds |
Similar to nonpolar but generally higher mp, bp, more likely to be water soluble. |
HCl
NH3 |
|
|
Network Covalent |
Atoms |
Covalent Bond |
--- |
Hard, very high-melting solids. Nonconductors. Insoluble in common solvents. |
C
SiO2 |
|
Ionic |
Ions |
--- |
Ionic Bonds |
High mp. Conductors in molten state or water solution. Often soluble in water, insoluble in organic solvents |
NaCl
MgO CaCO3 |
|
Metallic |
Cations |
--- |
Metallic Bonds |
Variable mp. Good conductors in solids. Insoluble in common solvents. |
Na
Fe |

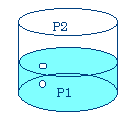
I. Liquid Vapor Equilibria
When a liquid is introduced into a closed container, it establishes equilibrium with its vapor:
Liquid ó vapor
The pressure of the vapor at equilibrium is referred to as the vapor pressure of the liquid
See p. 316 Know Figure 10-1
Nvapor = PV = (92/760 atm)(1.00L) ___= 0.0050mol
RT (0.0821 L atm/mol K)(298 K)Nliquid = 0.0100 – 0.0050 = 0.0050 mol
If the flask were larger than about 2L, all the liquid would vaporize, equilibrium would not be established.
Hence, boiling point varies with applied pressure, P2. When P2 = 760 mmHg, bp water = 100 0 C. If P2 = 1075 mmHg, bp water = 110 0 C (pressure cooker). If P2 = 5 mmHg, water boils at 00 C.
II. Phase Diagrams
Graph showing temperatures and pressures at which liquid, solid, and vapor phases of a substance can exist.
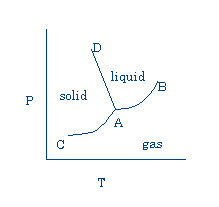
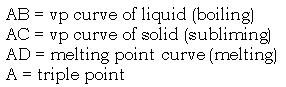
Note that:
- solid sublimes (passes directly to vapor) below triple point (00 C, 5mmHG for water; 1150 C, 90mmHg for I2 ).
- If line AD inclines toward P axis, melting point decreases as P increases. This behavior is observed for water, where the liquid is the more dense phase. More often, the solid is more dense, AD tilts away from the P axis, and the melting point increases with pressure. See p. 325.
The amount of heat absorbed when one mole of a solid sublimes is called the molar heat of sublimation. Applying Hess’s Law, we have, at a given temperature:
D
Hsubl = DHfus + DHvap(s à g) (sà l) (là g)
For water at 00Cm these quantities are 50.9, 6.0, and 44.9 kJ/mol, in that order. DHvap >> DHfus . The heat of sublimation, since it is the sum of the other two enthalpy changes, is the largest of the three.
III. Molecular Substances
Know acids HA ó H + + A –
There are 3 types of forces that hold molecular substances together [i.e. inter not intra] molecular.
See p. 327 in black.
Dispersion Forces (London Forces) Result from temporary dipoles formed in adjacent molecules. Strength depends upon how readily electrons are dispersed. Increase with molecular size, molar mass. Explains why boiling point of molecular substances ordinarily increases with molar mass. (F2 < Cl2 < Br2 < I2 ) Found in all molecular substances. See Figure 10.9. Be able to tell me a story about these. See second paragraph p. 328.
- Dipole Forces
Electrical attractive forces between + end of one polar molecule and – end of adjacent molecule.
Compare NO(bp = -151 0 C) to N2(bp =-196 0 C), O2 (bp =-183 0 C)
Be able to explain this, just like example 10.4 p.329.- Hydrogen Bonds
Unusually strong dipole force. H atom is very small and differs greatly in electronegativity from F, O, or N. Compare boiling points of Group 6 hydrides:
H2O (100 0 C) H2S (-61 0 C) H2Se (-42 0 C) H2Te (-2 0 C)
Note that water has many unusual properties in addition to high boiling point. Open structures of ice, a result of hydrogen bonding, accounts for its low density.
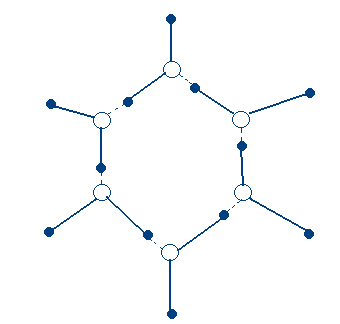
IV. Network Covalent Substances (C, SiC, SiO2) –Macromolecular
V. Ionic Substances (NaCl, KNO3)
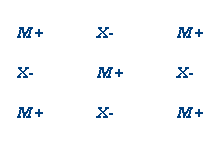
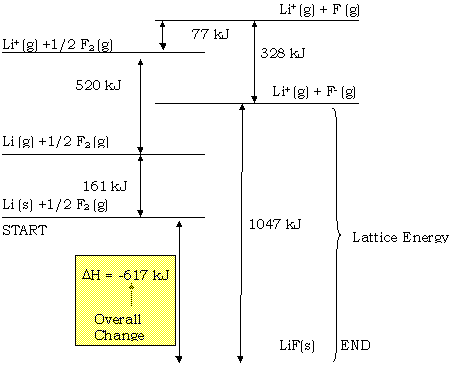
VI. MetalsLattice energy =
DH for the process occurring when separated gaseous ions are packed together to form an ionic solid. See p. 338.
What is CO2? Which has the higher MP? Why does it have a higher MP?
C2H3F?
C2H5COOH?
C2H5OH?
BaI2?
Ba?
SiO2?
An overview of molecular forces in relation to protein structure
An OK site on forces (if you still don't understand)
Back to the Class Notes Homepage
 |
|